CD46, discovered by Cole et al. in 1986 through C3b affinity chromatography on peripheral blood lymphocytes, is a membrane protein also known as membrane cofactor protein (MCP). While MCP shares homology with decay accelerating factor (DAF) in nucleotide sequence, their protein structures and functions differ, leading to CD46 being recognized as a new regulatory protein in the complement system. It was named CD46 during the Fourth International Workshop on Leukocyte Differentiation Antigens.
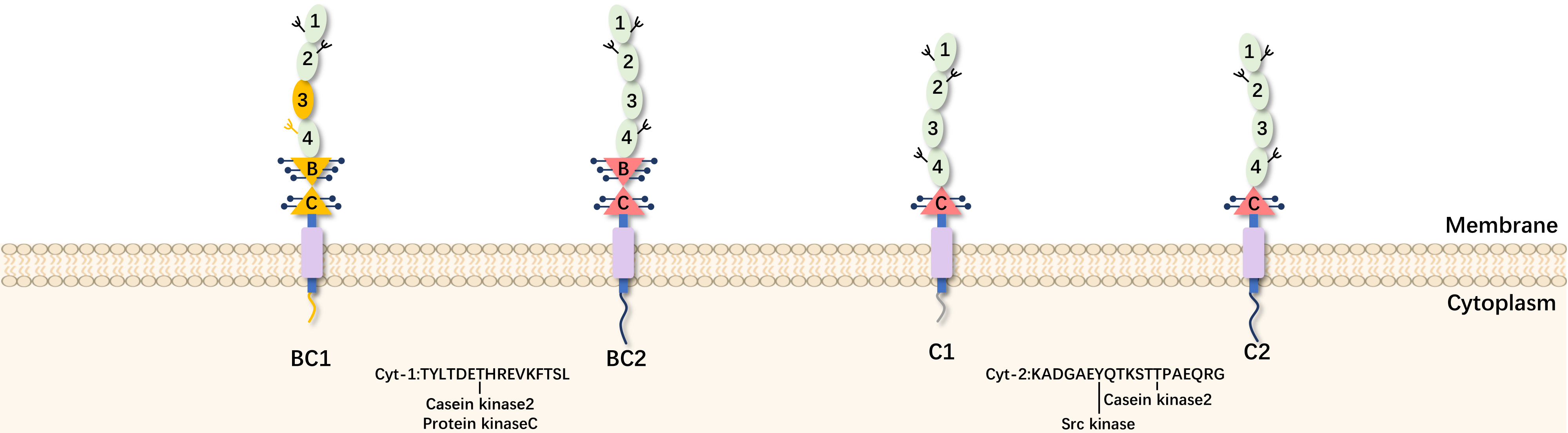
CD46 is a single-chain transmembrane glycoprotein located on the short arm of human chromosome 1 at the 32nd locus, with a molecular weight of 45-70 kDa, also referred to as gp45-70, belonging to the complement regulatory protein factor family. Due to the lack of exon A in the typical CD46 expression, the protein is classified into four subtypes: C1, C2, BC1, and BC2. BC subtypes have a higher molecular weight compared to the C subtypes.
Functioning as a cofactor for membrane-mediated C3b and C4b cleavage, CD46 serves as a key regulator in the classic and alternative complement activation cascades of the innate immune system. Excessive cleavage of C3b produces membrane-bound C3bi and soluble C3f, while C4b cleavage generates membrane-bound C4d and soluble C4c. Fragment binding to cells halts further complement cascade activation, safeguarding host cells against unintended complement-mediated lysis.