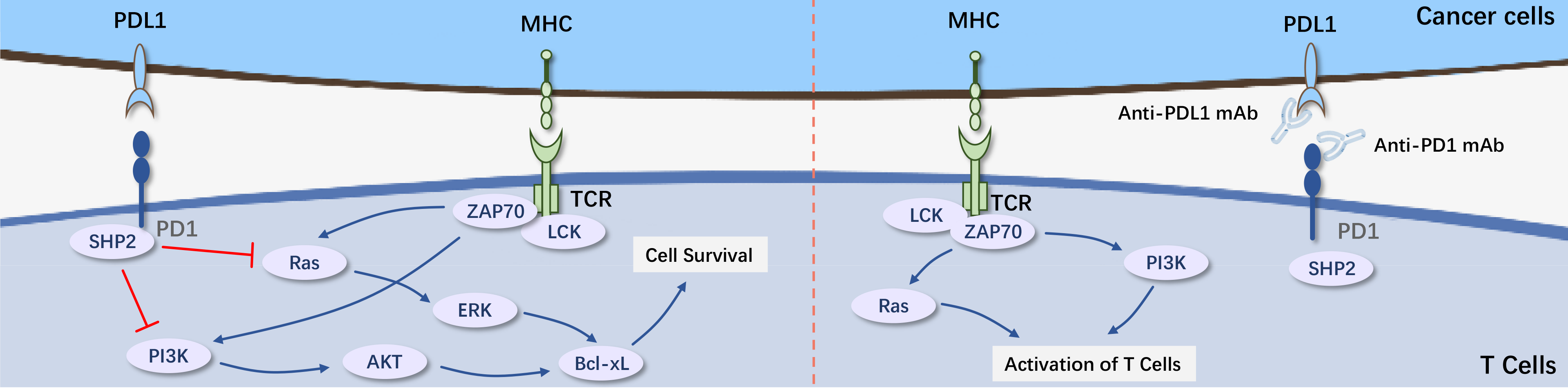
PD-1 is an immune inhibitory receptor expressed on activated T cells and B cells, playing a crucial role in regulating immune responses to tumor antibodies and self-antigens. The interaction between PD-1 on neighboring cells and its ligands PD-L1 or PD-L2 inhibits the transmission of the TCR signaling pathway, as well as TCR-mediated cell proliferation, transcription activation, and cytokine production.
PD-L1 monoclonal antibody is currently the hottest target in immunotherapy. By blocking the binding of PD-1 on activated T cells with PD-L1 on tumor cells, PD-L1 monoclonal antibody restores the body's immune cells' ability to effectively recognize tumors. O drug and K drug were approved for marketing in China in June and July 2018 respectively. Subsequently, Junshi Biosciences' Toripalimab and Cindat Biochemical's Sintilimab were approved for marketing in December 2018, Hengrui's Camrelizumab was approved in May 2019, BeiGene's Tislelizumab was approved in December 2019, and Kangfang Biotech's Pemigatinib and Yuheng Biotech's Saptinib were approved in August 2021.
PD-L1 monoclonal antibody has been approved for over twelve types of tumors, making it a well-deserved broad-spectrum anti-tumor drug. However, PD-L1 is not omnipotent, as primary and acquired resistance exist. Therefore, combination therapy with chemotherapy, other immune checkpoint inhibitors, monoclonal antibodies, and oncolytic viruses has become an inevitable trend. Furthermore, to improve response rates, PD-L1 expression level, microsatellite instability, and tumor mutation burden are important predictive biomarkers for efficacy.