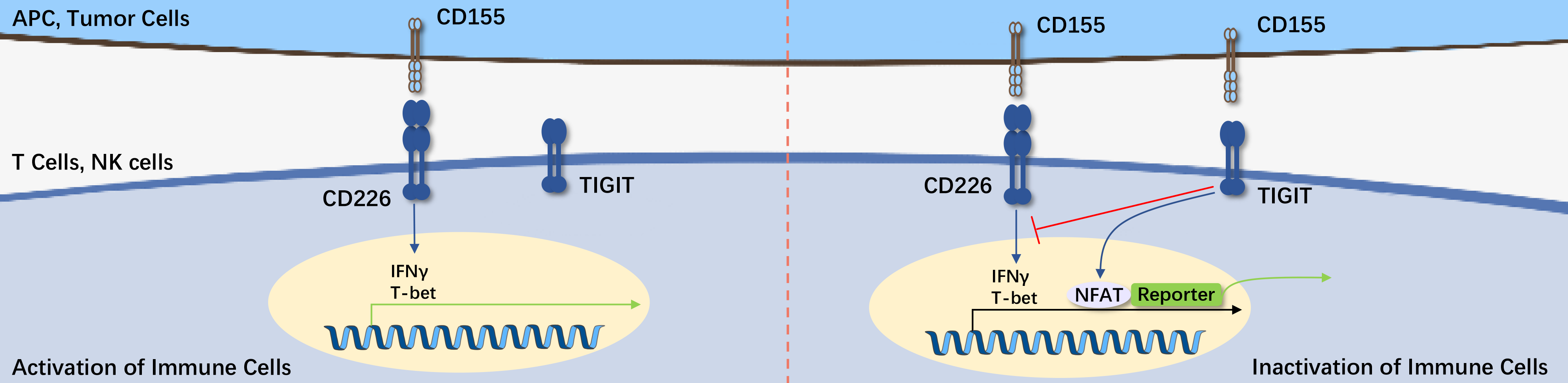
TIGIT (T cell Ig and ITIM domain) is a member of the Poliovirus Receptor (PVR)/Nectin family. It consists of an extracellular immunoglobulin variable (IgV) domain, a type 1 transmembrane domain, and intracellular domains with a classical immunoreceptor tyrosine-based inhibition motif (ITIM) and an immunoglobulin tyrosine tail (ITT) motif.
CD155 (PVR) is a high-affinity ligand for TIGIT. Once the tumor surface-overexpressed CD155 binds with TIGIT on the surface of NK and T cells, their cytotoxic effect on tumor cells is inhibited. Based on the principle of the TIGIT axis and the complex immune inhibitory patterns in the tumor microenvironment, how to safely and effectively block TIGIT in clinical practice has become a technical challenge that many medical researchers are eager to overcome, with the in vitro activity testing of Anti-TIGIT antibody drugs being the first step towards clinical application.
Building on these research findings, multiple TIGIT antibody developments have entered clinical trials. Roche's recently announced results from the Tiragolumab Phase II clinical trial showed that Tiragolumab, when combined with PD-L1 monoclonal antibody (Tecentriq), demonstrated higher objective response rate and progression-free survival compared to Tecentriq monotherapy in metastatic non-small cell lung cancer. The clinical trial has now progressed to Phase III to further validate the drug's treatment effects in a broader patient population. Additionally, several pharmaceutical companies, such as Merck MK-7694, Gilead AB154, and Bristol-Myers Squibb BMS-986207, are investing in the development of anti-TIGIT drugs, which are currently undergoing initial evaluations in Phase I/II clinical trials, with hopes of achieving success in the future.