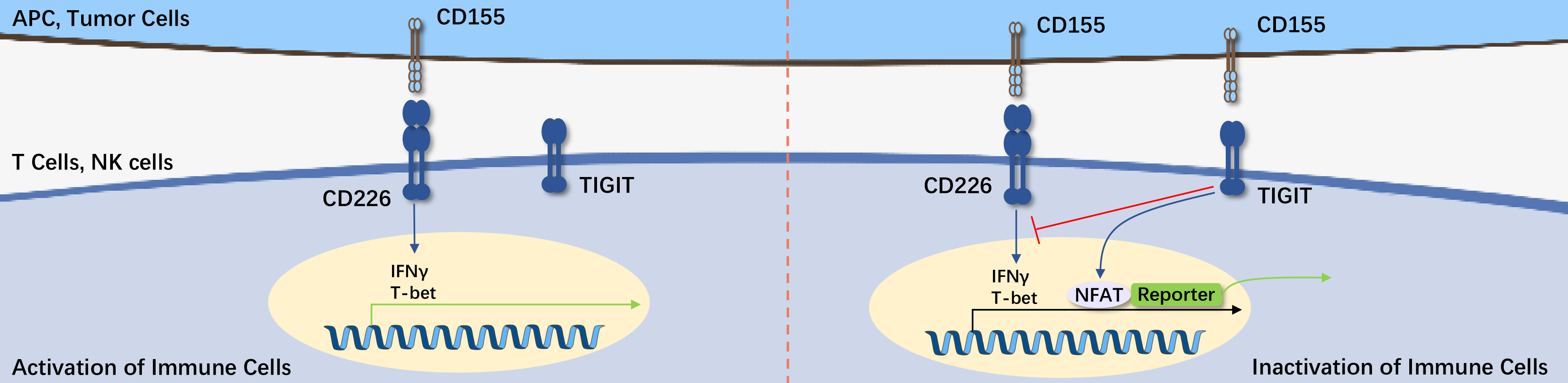
TIGIT (T cell Ig and ITIM domain) is a member of the poliovirus receptor (PVR)/Nectin family. It consists of an extracellular immunoglobulin variable domain (IgV), a type I transmembrane domain, and intracellular domains with classic immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoglobulin tyrosine tail (ITT) motifs.
CD155 (PVR) is a high-affinity ligand for TIGIT. Once the highly expressed CD155 on tumor surfaces binds to TIGIT on the surfaces of NK and T cells, their killing effect on tumor cells is inhibited. Based on the principle of the TIGIT axis and the complex immune suppression patterns in the tumor microenvironment, how to safely and effectively block TIGIT in clinical settings has become a technical challenge that many medical researchers aim to overcome, with the testing of anti-TIGIT antibody drugs being the first step towards clinical application.