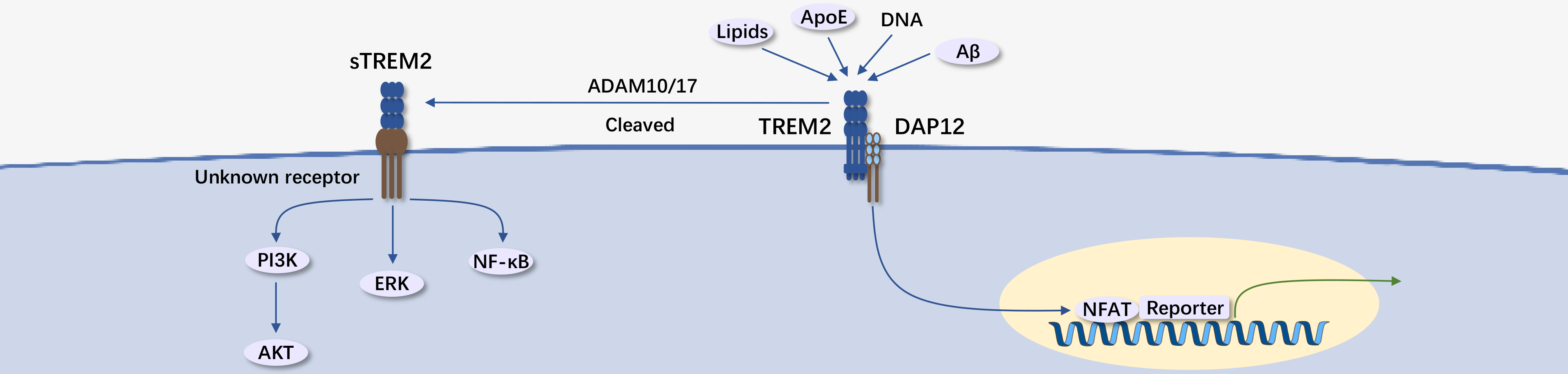
TREM2 belongs to the immunoglobulin superfamily and is a transmembrane receptor. TREM2 has multiple ligands, primarily negatively charged molecules that bind freely on the cell membrane. TREM2 has an extracellular domain consisting of an Ig-like V domain, a short extracellular domain, a transmembrane helical domain, and a short cytoplasmic tail, lacking signaling and transport motifs. In mouse macrophages, TREM2 can interact with adapter proteins DAP12 and DAP10. Upon ligand binding, the receptor molecules are phosphorylated, recruiting intracellular signaling molecules (DAP12 corresponding to Syk, DAP10 to PI3K).
The downstream signaling of TREM2 depends on the activity states of DAP10 and DAP12, determined by cell type and status. Low-affinity ligands can promote DAP12, inhibiting cell activation via SHP-1-Syk. In conditions like bacterial infection, pathogen invasion, tissue damage, and cell death, TREM2 binds to surface phospholipids and cell debris. In Alzheimer's disease brains, TREM2 can directly bind to pathological β-amyloid protein, forming plaques.
TREM2 signaling is emerging as a signaling hub in diseases like Alzheimer's, metabolic syndrome, and cancer. Numerous intervention strategies exist to target the TREM2 pathway for treatments. Notably, one approach involves using specific monoclonal antibodies or small molecules to target the receptor activation domain, thereby blocking or activating downstream signaling. Targeting TREM2 in Alzheimer's aims to activate the receptor, stimulate microglial cell phagocytosis, and clear amyloid deposits. Another potential strategy involves guiding tissue-specific TREM2 ligands to desired locations for activation.
In contrast, in cancer, TREM2's anti-inflammatory and immune-suppressive activities promote tumor growth and immune evasion. To combat this, blocking TREM2 signaling or depleting TREM2+ myeloid cells in tumors can activate T cell-mediated anti-tumor immune responses.