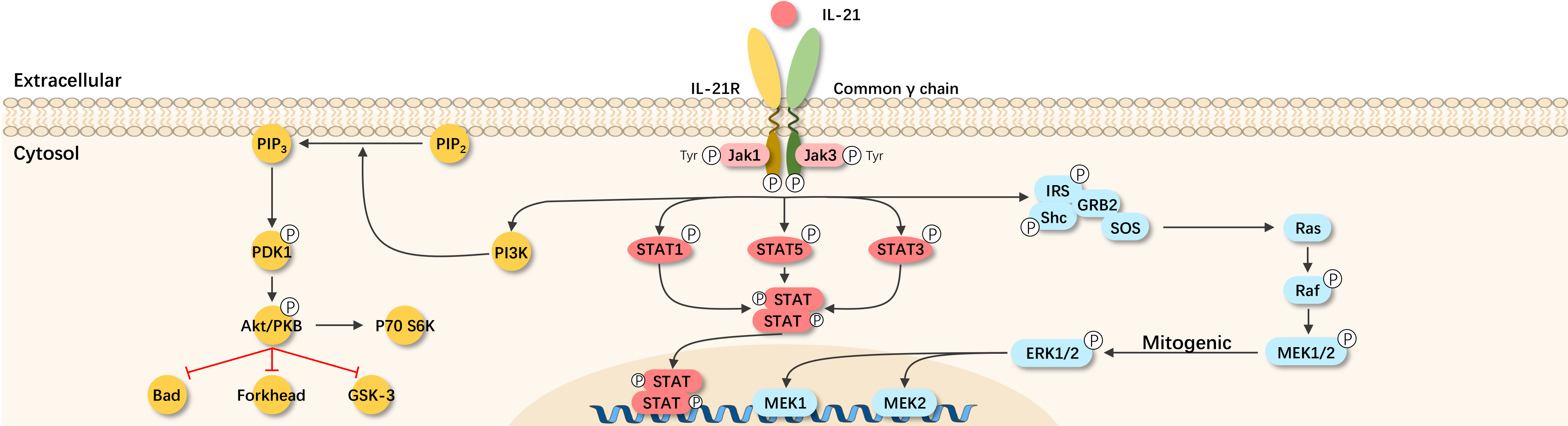
IL-21 is a cell factor with a four-helix bundle structure. The IL-21 receptor (IL-21R) is a heterodimer composed of unique subunits and the r chain, widely expressed on the surface of lymphocytes and hematopoietic cells, sharing the r chain with other members of the IL-2 family (IL-2, IL-4, IL-7, IL-9, IL-13, and IL-15). The IL-21R subunit is the ligand recognition binding site, and the r chain is the signal transduction unit. IL-21 signaling primarily occurs through the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathways. Once IL-21 binds to its receptor, it activates the JAK family members (JAK1 and JAK3), followed by phosphorylation of STAT1, STAT3, STAT4, and STAT5, ultimately regulating the expression of corresponding genes in the cell nucleus. Additionally, IL-21 can also activate members of the mitogen-activated protein kinase (MAPK) family.
An increasing amount of research indicates that IL-21 enhances the body's anti-tumor ability through its mechanisms in immune regulation, mainly achieved through the cytotoxic activity of NK cells and the effector function of CTLs. IL-21 promotes the differentiation and maturation of CD8+ T cells and NK cells, inducing these cells to secrete various active substances such as IFN-γ, perforins, and granzymes, inhibiting tumor cell growth, and enhancing anti-tumor capabilities. Cytokine therapy in clinical trials is a crucial part of cancer immunotherapy. Currently, IL-21 has undergone phase I and II clinical trials in patients with metastatic melanoma and renal cell carcinoma. Phase I clinical trial results indicate that the maximum tolerated dose of human IL-21 is 30 μg/kg, leading to activation of multiple cell and immune response pathways post-treatment.