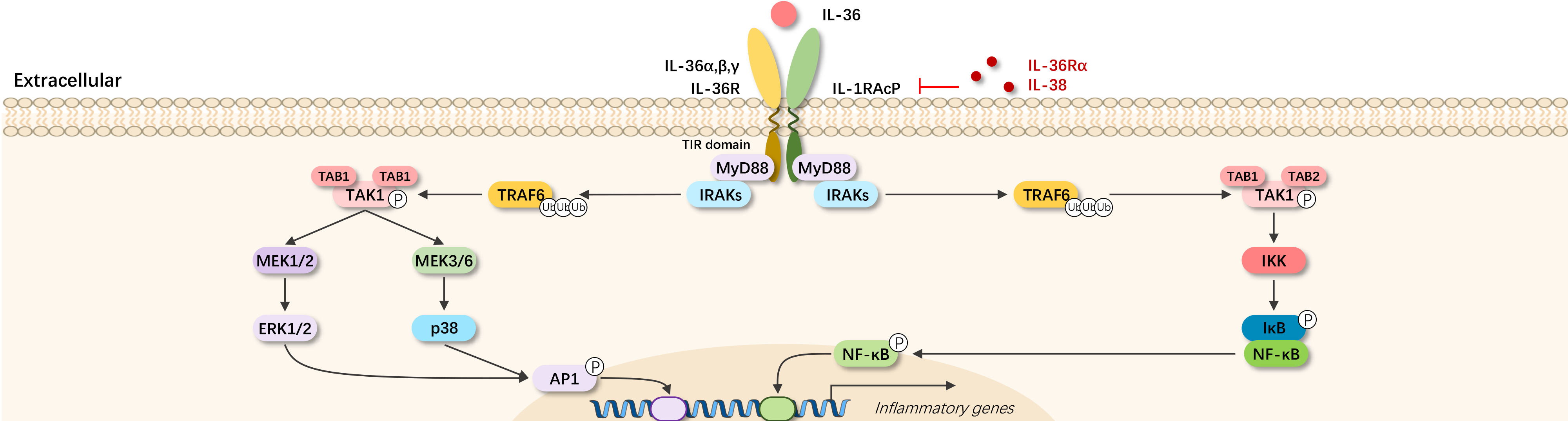
IL-36 was discovered in 1999, belonging to the IL-1 cytokine family. Once IL-36 undergoes N-terminal cleavage, it can become active and functional, playing important roles in inflammation and tumor immunity.
Currently, four receptor subtypes have been identified: IL-36α (IL-1F6), IL-36β (IL-1F8), IL-36γ (IL-1F9), and IL-36Rα (IL-1F5); the first three act as agonists of IL-36 receptors, promoting inflammation, while IL-36Rα functions as an antagonist, counteracting inflammation. The IL-36 receptors form heterodimers (IL-1R6, IL-1RAcP).
IL-36α, IL-36β, IL-36γ, when cleaved into their active forms, bind to IL-36 receptors, transmitting signals through the MyD88-MAPK/IKK-AP1/NF-κB pathway, leading to the release of cytokines (such as IL-36), chemokines, and inflammation promotion. The active form of IL-36Rα, unable to bind to IL-36 receptors, does not signal, effectively antagonizing inflammation.