On June 30, 2025, Amgen announced that bemarituzumab, an FGFR2b-targeted antibody co-developed with Zai Lab, met its primary endpoint of overall survival (OS) early in the phase 3 FORTITUDE-101 trial as a first-line treatment for gastric cancer. This milestone highlights FGFR2b as a key oncogenic driver in gastric and other types of cancer.
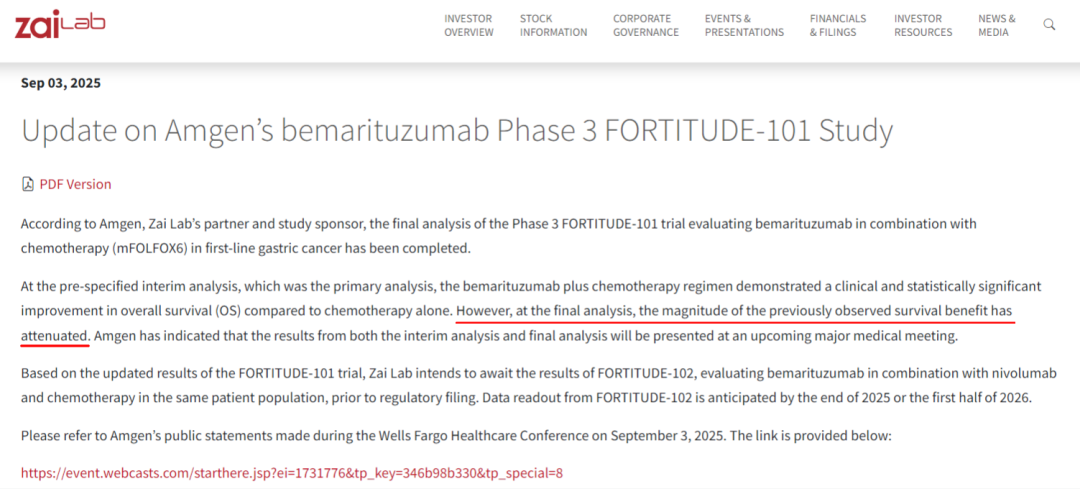
On September 3, Zai Lab released an update on the FORTITUDE-101 phase 3 study of bemarituzumab, conducted in partnership with Amgen. The interim analysis demonstrated that bemarituzumab in combination with chemotherapy (mFOLFOX6) achieved a significant and clinically meaningful improvement in overall survival (OS) compared with chemotherapy alone. Although the survival benefit of bemarituzumab was slightly reduced in the final analysis, the overall data continue to support the clinical value of targeting FGFR2b in gastric cancer treatment.
With the advancement of related studies, the FGFR family—particularly the FGFR2b subtype—is increasingly recognized as a promising breakthrough target in the treatment of epithelial tumors.
FGFR Family: Key Regulators of Cellular Signaling
FGFR (Fibroblast Growth Factor Receptor) is a class of transmembrane receptor tyrosine kinases, comprising four members: FGFR1–FGFR4. They play a central role in development, tissue repair, and cell growth.
Each FGFR consists of three extracellular immunoglobulin-like domains (Ig I–III), a single transmembrane domain, and an intracellular tyrosine kinase domain. Upon binding with fibroblast growth factors (FGFs) and subsequent receptor dimerization, the kinase domain is phosphorylated, activating multiple downstream signaling pathways, including RAS–MAPK/ERK, PI3K–AKT, PLCγ–PKC, and STAT (Dai et al., 2019). These pathways collectively regulate cell proliferation, migration, differentiation, and survival.
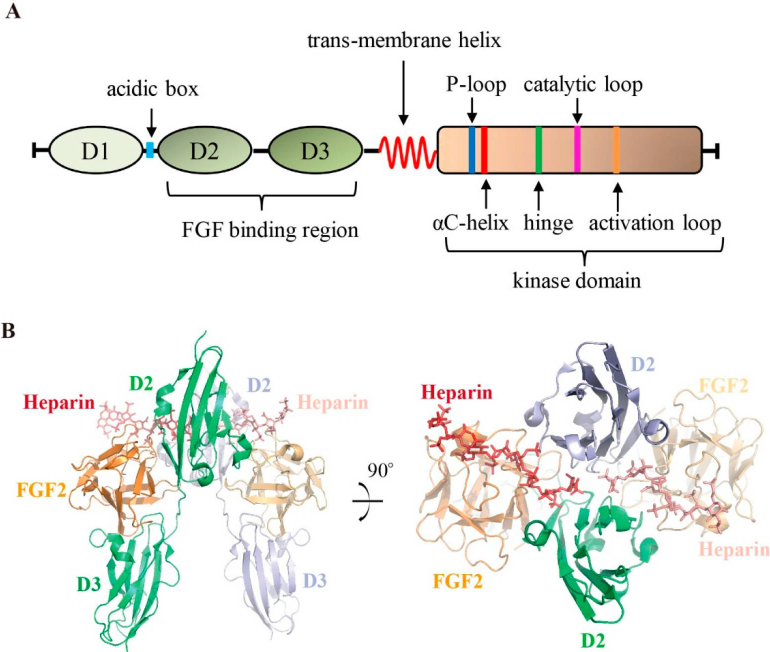
Under normal physiological conditions, the FGF–FGFR signaling axis maintains homeostatic balance between the epithelium and stroma. However, when this system is dysregulated—such as through FGFR gene amplification, mutation, fusion, or overexpression—the receptor becomes constitutively active. This results in excessive activation of downstream signaling pathways, promoting sustained cell proliferation and resistance to apoptosis. Such dysregulation is a key molecular mechanism driving the initiation and progression of various cancers.
Among them, FGFR2b is a splice variant of FGFR2, predominantly expressed in epithelial cells. Its aberrant activation can drive the development of epithelial-derived cancers, including gastric, lung, and breast cancers.
Global Status of FGFR-Targeted Drugs
Currently, four FGFR-targeted drugs have been approved worldwide: Pemigatinib, Erdafitinib, Infigratinib, and Futibatinib. All of these drugs target tumors associated with FGFR gene mutations or fusions, with particularly notable efficacy in FGFR2 fusion-positive cholangiocarcinoma.
Pemigatinib
Pemigatinib is the world’s first targeted therapy approved for cholangiocarcinoma. In patients with FGFR2 fusion-positive cholangiocarcinoma, pemigatinib achieved an objective response rate (ORR) of 36% and a median duration of response (DoR) of 7.49 months. It was approved for this indication in the United States in April 2020 and in China in April 2022. Beyond cholangiocarcinoma, pemigatinib has also been approved in the U.S. and Japan for the rare disease 8p11 myeloproliferative syndrome and is under investigation for multiple solid tumors, including non-small cell lung cancer, endometrial cancer, and bladder cancer.
Erdafitinib
Erdafitinib is the first FGFR-targeted drug approved globally. It received FDA approval in 2019 for the treatment of patients with advanced urothelial carcinoma harboring FGFR2 or FGFR3 alterations. In a recent pan-cancer study, erdafitinib demonstrated antitumor activity across 16 different tumor types, with ORRs exceeding 50% in patients with pancreatic cancer and cholangiocarcinoma. Erdafitinib has also submitted a marketing application in China, potentially offering a new treatment option for domestic patients.
Futibatinib
Futibatinib is an irreversible covalent FGFR inhibitor, mainly used for cholangiocarcinoma with FGFR2 fusions or rearrangements. Compared with reversible inhibitors such as pemigatinib, futibatinib binds more firmly to its target. In a phase II clinical trial, futibatinib achieved an ORR of 42%, a median DoR of 9.7 months, and a median overall survival approaching two years, demonstrating durable and significant antitumor activity.
Building on the currently approved FGFR-targeted drugs, researchers are continuously expanding therapeutic strategies targeting FGFR2, with a particular focus on precision therapies for tumors overexpressing FGFR2b.
Current investigational FGFR-targeted drugs include various types, such as small-molecule inhibitors and monoclonal antibodies. These agents are being evaluated both as monotherapies and in combination with chemotherapy or immunotherapy to further enhance treatment efficacy.
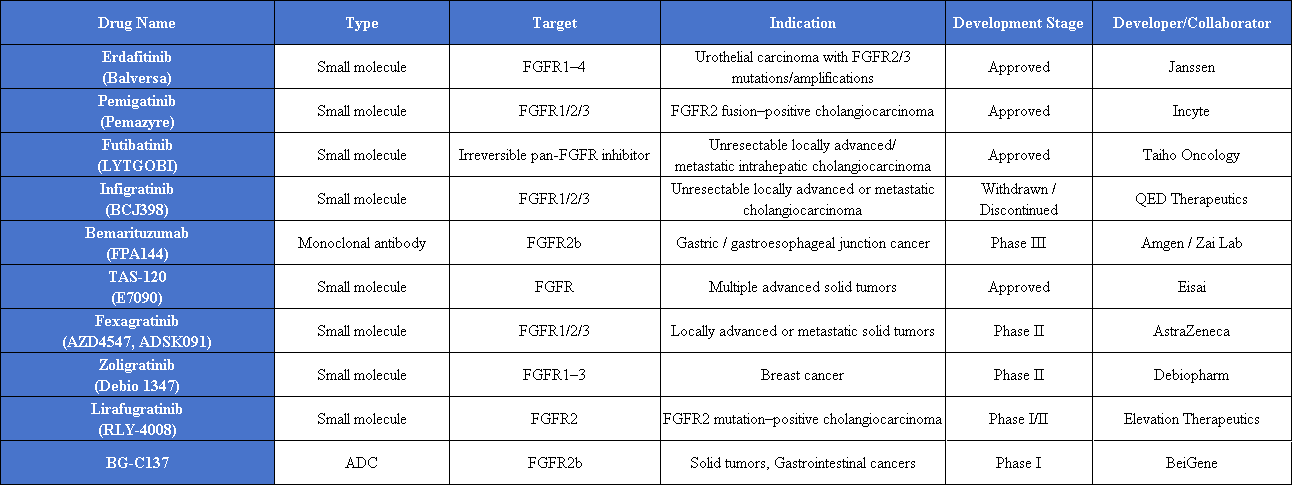
Among monoclonal antibody–based therapies, bemarituzumab has shown the fastest progress. Bemarituzumab, an FGFR2b-targeted monoclonal antibody, is being co-developed by Zai Lab and Amgen and is currently in Phase III clinical development globally.
As an FGFR2b-targeted monoclonal antibody, bemarituzumab is designed to inhibit tumor cell proliferation by blocking aberrant FGFR2b-mediated signaling. Globally, bemarituzumab is being evaluated in multiple clinical trials for its efficacy and safety in gastric cancer and other solid tumors.
On June 30, 2025, Amgen announced that the combination of bemarituzumab with mFOLFOX6 chemotherapy met the primary endpoint of overall survival (OS) early in the Phase III FORTITUDE-101 trial. FORTITUDE-101 is a randomized, multicenter, double-blind, placebo-controlled Phase III study designed to evaluate the efficacy of bemarituzumab combined with chemotherapy in patients with FGFR2b-positive advanced gastric cancer. In a pre-specified interim analysis, bemarituzumab plus mFOLFOX6 demonstrated a significant improvement in OS, successfully meeting the primary endpoint.
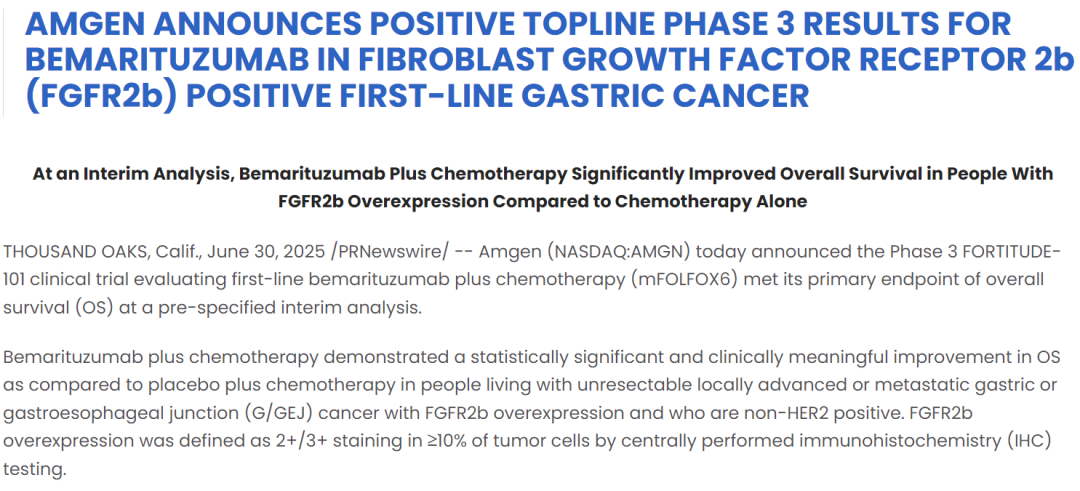
However, in the latest data update on September 3, 2025, Amgen and Zai Lab released the final analysis results of the FORTITUDE-101 trial. Compared with the interim analysis, the final analysis showed a slightly reduced OS benefit for bemarituzumab combined with chemotherapy. Nevertheless, Amgen stated that detailed efficacy and safety data will be presented at an upcoming major medical conference to further assess the clinical value of the regimen.
In addition, the FORTITUDE-101 trial evaluated the safety of bemarituzumab combined with chemotherapy. Common adverse events included blurred vision, punctate keratitis, anemia, neutropenia, nausea, corneal epithelial defects, and dry eye. The frequency and severity of ophthalmic-related adverse events were higher in the bemarituzumab group compared with the placebo group, indicating the need for enhanced ophthalmologic monitoring during clinical use.
Next-Generation FGFR2b ADCs and Biologic Drug Development
With the clinical progress of bemarituzumab, industry interest in FGFR2b-targeted therapies has further increased, and antibody-drug conjugates (ADCs) have emerged as a popular direction. Multiple companies worldwide are accelerating the development of next-generation FGFR2b ADCs, aiming to expand the therapeutic window while balancing efficacy and safety.
For example, BG-C137, developed by BeiGene, is an FGFR2b-targeted ADC with a topoisomerase I (TOPOI) inhibitor payload and a drug-to-antibody ratio (DAR) of 8. It employs a stable linker designed to potentially reduce corneal toxicity and has advanced to Phase I clinical trials.
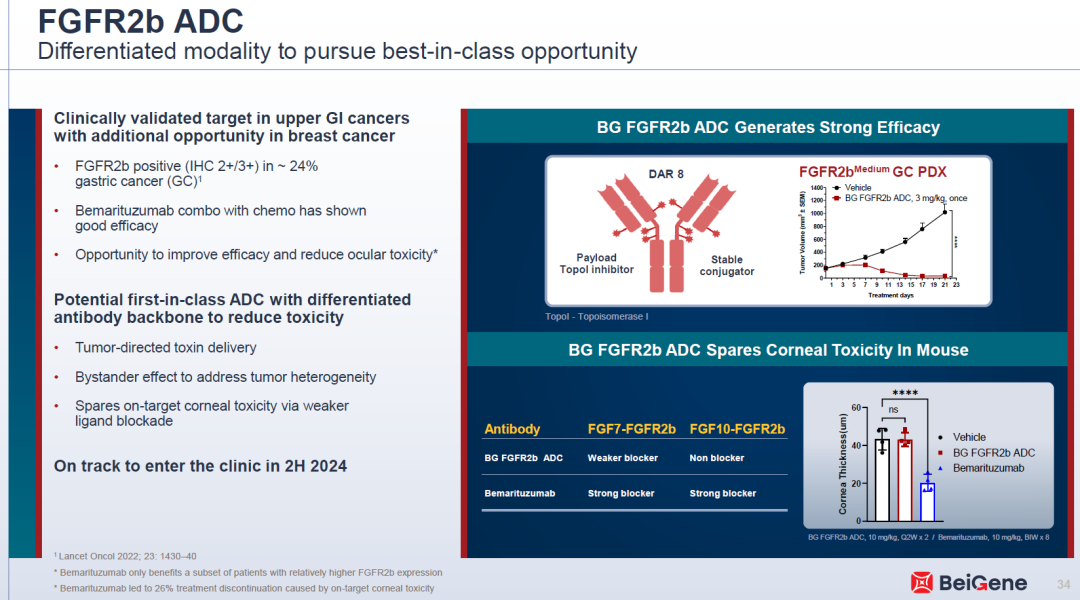
In addition, several early-stage FGFR ADC candidates have been designed with optimized antibody epitopes and linkers to reduce ocular toxicity associated with FGF10 inhibition, while leveraging advanced payload platforms to enhance both safety and efficacy. These strategies will be further validated in upcoming clinical trials.
It is anticipated that, with continued innovation and clinical validation, FGFR-targeted therapies have the potential to become a central component of next-generation cancer treatment, offering patients meaningful hope and transformative benefits.
Reference
AstraZeneca. (2015). AstraZeneca immuno-oncology pipeline combination treatments at ASCO 2015. https://www.astrazeneca.com/media-centre/press-releases/2015/astrazeneca-immuno-oncology-pipeline-combination-treatments-asco-2015-13052015.html
Dai, S., Zhou, Z., Chen, Z., Xu, G., & Chen, Y. (2019). Fibroblast growth factor receptors (FGFRs): Structures and small molecule inhibitors. Cells, 8(6), 614. https://doi.org/10.3390/cells8060614
ElevateBio. (n.d.). Lirafugratinib. ElevateBio. https://elevartx.com/lirafugratinib/
Eisai Co., Ltd. (2024). Eisai announces results from clinical study. https://www.eisai.com/news/2024/news202486.html
Food and Drug Administration. (n.d.). FDA approves erdafitinib for locally advanced or metastatic urothelial carcinoma. U.S. Food and Drug Administration. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-erdafitinib-locally-advanced-or-metastatic-urothelial-carcinoma
Food and Drug Administration. (n.d.). Withdrawn: FDA grants accelerated approval to infigratinib for metastatic cholangiocarcinoma. U.S. Food and Drug Administration. https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma
Incyte Corporation. (n.d.). FDA approves Incyte’s Pemazyre™ (pemigatinib), the first targeted treatment for previously treated, locally advanced or metastatic cholangiocarcinoma with an FGFR2 fusion or rearrangement. https://investor.incyte.com/news-releases/news-release-details/fda-approves-incytes-pemazyretm-pemigatinib-first-targeted
Amgen Inc. (2025). Amgen announces positive topline Phase 3 results for bemarituzumab in fibroblast growth factor receptor 2b (FGFR2b)-positive first-line gastric cancer. https://www.amgen.com/newsroom/press-releases/2025/06/amgen-announces-positive-topline-phase-3-results-for-bemarituzumab-in-fibroblast-growth-factor-receptor-2b-fgfr2b-positive-firstline-gastric-cancer
Zymeworks Inc. (2025). Update on Amgen’s bemarituzumab Phase 3 FORTITUDE-101 study. https://zailab.gcs-web.com/news-releases/news-release-details/update-amgens-bemarituzumab-phase-3-fortitude-101-study/
Taiho Oncology, Inc. (2022). FDA approval of Lytgobi (futibatinib) for previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma with FGFR2 fusion or rearrangement. https://www.taihooncology.com/us/news/2022-09_lytgobi_approval/
The ASCO Post. (2019). Clinical trial information: FORTITUDE-101 (bemarituzumab) Phase 3 study in gastric cancer. Journal of Clinical Oncology, 37(15_suppl), TPS3157. https://ascopubs.org/doi/10.1200/JCO.2019.37.15_suppl.TPS3157
U.S. National Library of Medicine. (n.d.). A first-in-human study of BG-C137, an anti-FGFR2b monoclonal antibody, in patients with advanced solid tumors (NCT06625593). ClinicalTrials.gov. https://www.clinicaltrials.gov/study/NCT06625593