Recently, VelaVigo Bio made a significant breakthrough in the development of TSHR-targeted therapies. Its core bispecific antibody, VBS-102 (OLN102), was licensed to the U.S. biotechnology company Ollin Biosciences for USD 440 million. Subsequently, Ollin Biosciences completed a USD 100 million Series A financing round to support the global development of this product.
The clinical and industrial breakthroughs of TSHR stem from its pivotal role in regulating thyroid function. The thyroid-stimulating hormone receptor (TSHR) is the primary receptor for thyroid-stimulating hormone (TSH) and thyrostimulin. By controlling the synthesis and secretion of thyroid hormones, it maintains endocrine homeostasis in the body. This biological characteristic not only establishes a scientific basis for TSHR as a therapeutic target but also guides the development of various innovative drugs.
TSHR: The Core Receptor in Thyroid Function Regulation
TSHR is a cell-surface glycoprotein receptor belonging to the leucine-rich repeat-containing (LGR) subfamily of G protein-coupled receptors (GPCRs).
The TSHR gene contains 10 exons. The first nine exons encode the extracellular domain (ECD) from the N-terminus, while the tenth exon encodes the seven transmembrane helices and the C-terminal region containing the intracellular domain. The TSHR gene encodes a full-length protein of 764 amino acid residues, with a molecular weight of 87 kDa.
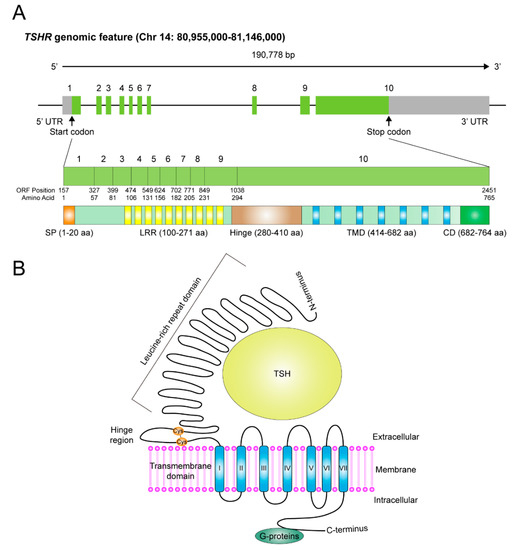
TSHR is primarily distributed on the basolateral membrane of thyroid follicular cells, and research on it has been extensive. Expression and functional roles of TSHR have also been reported in various non-thyroid cancer tissues, including melanoma, glioma, lung cancer, breast cancer, ovarian cancer, and liver cancer.
TSHR Signaling Pathway Mechanism
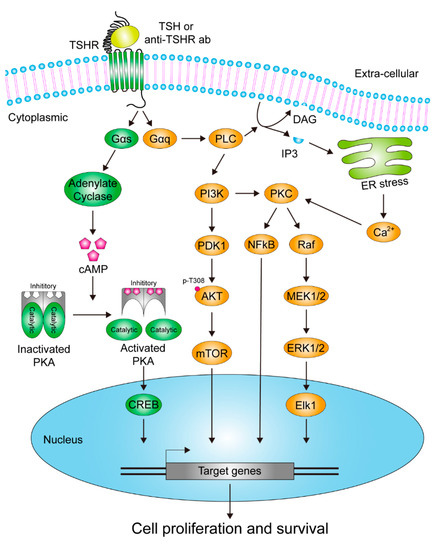
As a class A G protein-coupled receptor (GPCR), TSHR is activated by multiple ligands in thyroid physiology and regulates thyroid cell growth and hormone synthesis through classical downstream G protein signaling cascades. Its activation is multi-faceted: in addition to the physiological ligand circulating thyroid-stimulating hormone (TSH), it can also be triggered by stimulating autoantibodies in autoimmune diseases, gain-of-function mutations that lead to constitutive receptor activity, and a high-affinity ligand known as thyroid-stimulating protein.
Key downstream signaling pathways:
Gαs Pathway: Activation of the adenylate cyclase–cAMP–protein kinase A (PKA) axis leads to phosphorylation of the transcription factor CREB, driving the expression of genes involved in hormone synthesis and cell proliferation.
Gαq Pathway: Activation of phospholipase C (PLC) generates IP3 and DAG, which activate protein kinase C (PKC), and further engage MAPK and PI3K-AKT signaling cascades, collectively promoting cell proliferation and survival.
These two pathways do not operate independently but form a finely tuned, coordinated regulatory network. The Gαs/cAMP/PKA pathway mainly regulates thyroid cell differentiation and specific functions, such as hormone production, whereas the Gαq/PLC/PKC pathway primarily drives proliferation and survival signals. The coordination and balance between these pathways are critical for maintaining normal thyroid physiology, supporting hormone secretion, and controlling tissue growth. Dysregulation of this network is a central mechanism underlying hyperthyroidism, goiter, and thyroid cancer.
Research Progress on TSHR-Targeted Drugs
With a deeper understanding of the biological characteristics of the thyroid-stimulating hormone receptor (TSHR), drug development targeting this receptor has successfully expanded from traditional endocrine therapies to include cancer immunotherapy and autoimmune disease treatments, forming a diversified therapeutic landscape.
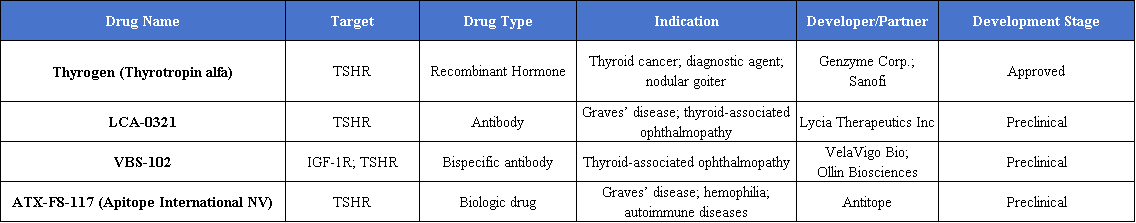
In the diagnosis and treatment of thyroid cancer, recombinant human thyroid-stimulating hormone (rhTSH) serves as a standard adjuvant therapy. By effectively elevating serum TSH levels, it significantly enhances the clearance of thyroid tissue during radioactive iodine therapy and provides an important basis for postoperative monitoring. A notable example is Thyrogen by Sanofi, which was first approved in 1998.
In the field of autoimmune disease treatment, therapeutic strategies for Graves’ disease and its associated thyroid eye disease (TED) primarily focus on blocking the abnormal activation of TSHR signaling pathways. VelaVigo Bio has made a significant breakthrough in this area with its IGF-1R/TSHR bispecific antibody, VBS-102 (also known as OLN102). In April 2025, the antibody was licensed to U.S. biotechnology company Ollin Biosciences in an exclusive overseas deal valued at USD 440 million. By simultaneously targeting IGF-1R and TSHR, VBS-102 achieves synergistic blockade of pathogenic signaling. The antibody is expected to enter clinical development in 2026 and holds promise for offering improved safety and efficacy for TED patients.
Summary and Outlook
The development of TSHR-targeted therapies clearly illustrates the evolution of treatment concepts—from hormone replacement to immune modulation, and from single-target approaches to multi-mechanism synergistic strategies. As more innovative drugs advance through clinical development, TSHR will continue to provide thyroid disease patients with more effective and precise therapeutic options.
Reference List
Food and Drug Administration. (1998). Thyrogen (thyrotropin alfa for injection) [Approval letter, printed labeling, medical review]. U.S. Department of Health & Human Services. https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20898_Thyrogen.cfm
Lycia Therapeutics. (n.d.). Pipeline: Programs. https://lyciatx.com/pipeline/programs/
PR Newswire. (2025). Velavigo announces second out-licensing deal of a first-in-class bi-specific antibody, further validating its innovative discovery platform and sustainable BDVC business model. https://www.prnewswire.com/news-releases/velavigo-announces-second-out-licensing-deal-of-a-first-in-class-bi-specific-antibody-further-validating-its-innovative-discovery-platform-and-sustainable-bdvc-business-model-302431714.html
PR Newswire. (2025). FDA grants orphan status for ATX-F8-117 for the treatment of haemophilia A patients in the US. https://www.prnewswire.com/news-releases/fda-grants-orphan-status-for-atx-f8-117-for-the-treatment-of-haemophilia-a-patients-in-the-us-506593851.html