Once a prominent target in hematologic malignancies, BCMA is now leveraging its unique biological characteristics to rapidly expand from oncology into autoimmune disease, initiating a new wave of immune modulation.
In June of this year, Harbour BioMed and Otsuka Pharmaceutical entered a global strategic collaboration to jointly advance the development of HBM7020, a BCMA×CD3 bispecific T-cell engager for autoimmune diseases. Over the past year, from Oncimmune Biotech and Connoia to Genrix Bio, four BCMA/CD3 bispecific antibodies have been developed for autoimmune indications within just ten months, highlighting BCMA as an emerging hotspot.
BCMA Overview: From Plasma Cell Biology to Targeted Therapy
B-cell maturation antigen (BCMA, also known as TNFRSF17 or CD269) is a member of the tumor necrosis factor receptor (TNFR) superfamily and a critical transmembrane glycoprotein for maintaining plasma cell fate.
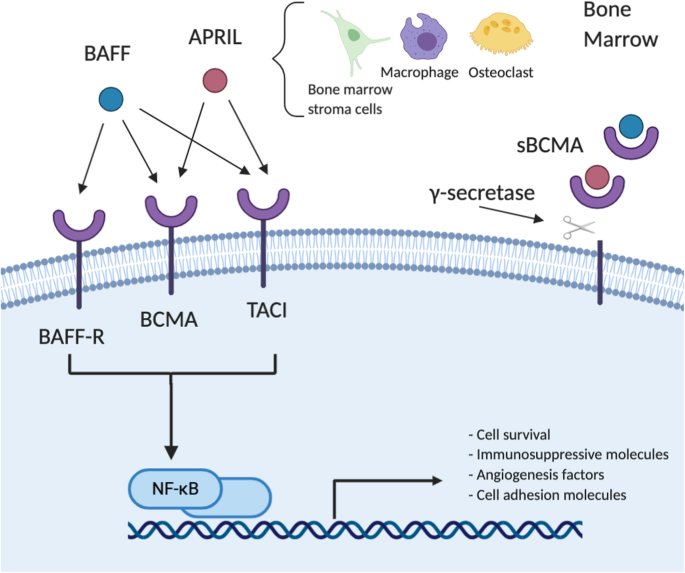
BCMA’s primary ligands include two other members of the TNFR superfamily—A Proliferation-Inducing Ligand (APRIL) and B-cell Activating Factor (BAFF), with APRIL exhibiting higher affinity for BCMA.
BCMA is minimally expressed in hematopoietic stem cells and other non-immune tissues but is highly expressed in mature B cells, especially long-lived plasma cells (LLPCs), where it plays a key role in survival and anti-apoptotic regulation. In multiple myeloma (MM), BCMA is highly expressed on malignant plasma cells in all patients, while normal tissue expression remains very low, providing the rare combination of high selectivity and a favorable safety window.
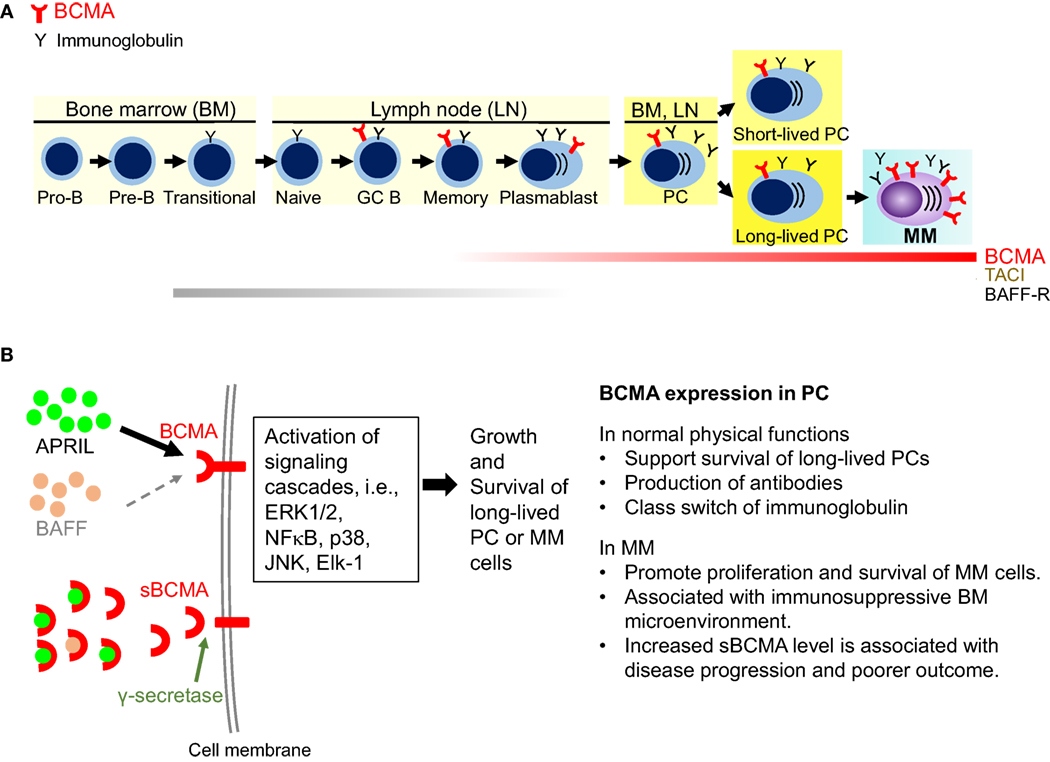
Upon binding with APRIL or BAFF, BCMA activates multiple signaling pathways, primarily NF-κB, MAPK, and PI3K/Akt. These pathways collectively promote upregulation of anti-apoptotic proteins, enhance cell proliferation, and induce the expression of adhesion molecules, angiogenic factors, and immunosuppressive molecules, thereby creating a microenvironment that supports the sustained survival of plasma cells, particularly myeloma cells. Specifically, BCMA activation triggers nuclear factor κB (NF-κB) in B cells, supporting cell proliferation and survival.
BCMA also interacts with molecules such as CD138, heparan sulfate proteoglycans (HSPGs), and the transcription factor IRF-4, further reinforcing its central role in plasma cell biology. Meanwhile, a portion of membrane-bound BCMA can be cleaved into soluble BCMA (sBCMA), which competitively binds APRIL and BAFF, thereby modulating and attenuating BCMA signaling to some extent.
With its highly specific tissue expression and critical role in plasma cell survival, BCMA has become one of the most representative targets in multiple myeloma and provides a solid biological foundation for its application in autoimmune diseases.
Genomeditech has independently developed a series of BCMA-related products, including stable overexpression cell lines, reporter gene assay cell lines, antibodies, and protein-based products, fully supporting drug development needs and accelerating clinical translation.
BCMA in Autoimmune Disease Development
The development of novel BCMA-targeting therapeutics is progressing rapidly, with multiple treatment modalities, including CAR-T cells, multispecific antibodies/T-cell engagers (bispecifics, trispecifics, BiTEs, etc.), antibody-drug conjugates, and cancer vaccines.
CAR-T cell therapy is one of the most actively developed BCMA-targeted approaches and has shown significant efficacy. On October 16, 2025, IASO Biologics, a company focused on innovative cell therapies, published in the international journal Cell its research on the fully human BCMA-targeted CAR-T therapy Iqocelucel for treating progressive multiple sclerosis (PMS).
The study demonstrated the safety and efficacy of CAR-T therapy in progressive multiple sclerosis and represents Cell’s first publication of BCMA-targeted CAR-T therapy applied to an autoimmune disease, marking a significant scientific advancement in this field.
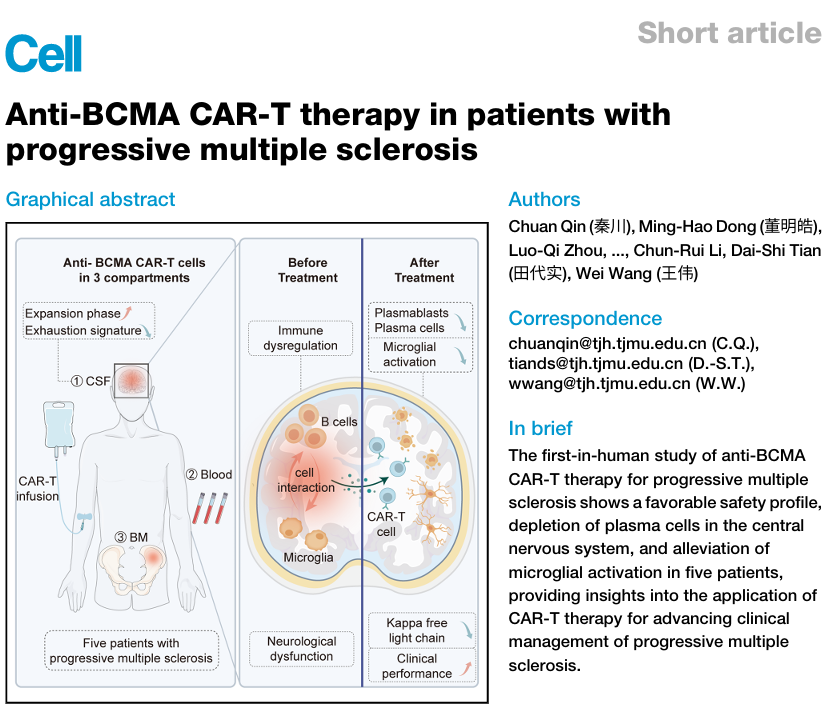
Based on BCMA’s success in multiple myeloma, its potent therapeutic effect and favorable safety profile as an immunotherapy target have been established. Several clinical trials are currently evaluating the safety and efficacy of BCMA-targeting products in autoimmune diseases.
For example, in systemic lupus erythematosus (SLE), a study titled B-Cell Maturation Antigen (BCMA) as a Biomarker and Potential Treatment Target in Systemic Lupus Erythematosus reported that BCMA expression was generally elevated in B-cell subsets of SLE patients and positively correlated with the proportion of plasmablasts and serum autoantibody levels. These findings indicate that BCMA could serve not only as a biomarker for disease activity but also as a potential therapeutic target.
Additionally, in the study by Sanges et al., patients with systemic sclerosis (SSc) who had pulmonary arterial hypertension exhibited higher soluble BCMA (sBCMA) levels compared with other study participants (p = 0.03), further supporting the feasibility of BCMA-targeted therapy.
Although BCMA-targeted CAR-T cell therapy remains a major focus, BCMA bispecific and trispecific antibodies have emerged as promising alternative treatments.
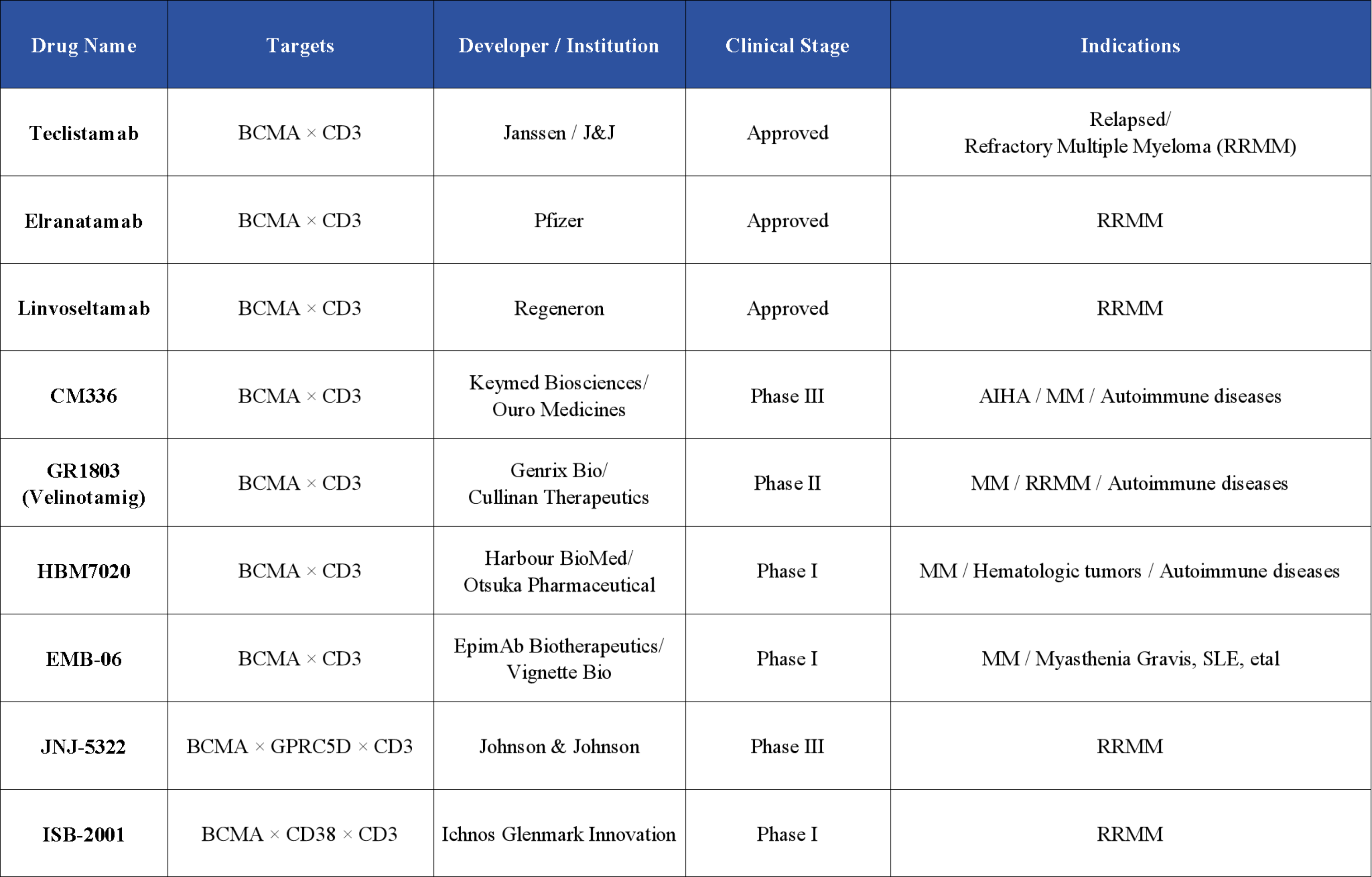
Bispecific antibodies bind simultaneously to BCMA and T-cell surface antigens, bringing the two cell types into close proximity and enhancing T-cell–mediated cytotoxicity against tumor cells. In the BCMA bispecific antibody field, three drugs—Teclistamab, Elranatamab, and Linvoseltamab—have been approved for the treatment of relapsed/refractory multiple myeloma (RRMM). Teclistamab is the first FDA-approved BCMA-targeted bispecific antibody and the world’s first BCMA/CD3 bispecific antibody.
For trispecific antibodies, clinical development is actively ongoing: JNJ-5322 (Johnson & Johnson) has entered Phase III clinical trials for RRMM; ISB-2001 (Ichnos Glenmark Innovation) are in Phase I trials, targeting RRMM.
Antibody-drug conjugates (ADCs) combine antibodies with cytotoxic agents to deliver drugs directly to malignant plasma cells via BCMA targeting. For example, Belantamab mafodotin (Blenrep) has been approved by the FDA and has significantly improved outcomes in multiple myeloma. Belantamab mafodotin is currently the only FDA-approved BCMA-targeted ADC.
Additionally, several BCMA bispecific T-cell engagers under development—such as CM336, GR1803 (Velinotamig), HBM7020, and EMB-06—are being explored not only for MM/RRMM but also for new indications including autoimmune diseases.
Summary
With its highly specific expression and favorable safety profile, BCMA is expanding from hematologic malignancies into autoimmune diseases. Multiple therapeutic strategies—including CAR-T cells, bispecific antibodies, and ADCs—are under development, advancing the field of immune-targeted therapies.
Reference List
Cho, S. F., Anderson, K. C., & Tai, Y. T. (2018). Targeting B cell maturation antigen (BCMA) in multiple myeloma: Potential uses of BCMA-based immunotherapy. Frontiers in Immunology, 9, 1821. https://doi.org/10.3389/fimmu.2018.01821
Shah, N., Chari, A., Scott, E., et al. (2020). B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia, 34, 985–1005. https://doi.org/10.1038/s41375-020-0734-z
Cell Press. (2025). [Title not provided]. Cell. https://www.cell.com/cell/fulltext/S0092-8674(25)01088-8
Martin, J., Cheng, Q., Laurent, S. A., Thaler, F. S., Beenken, A. E., Meinl, E., Krönke, G., Hiepe, F., & Alexander, T. (2024). B-cell maturation antigen (BCMA) as a biomarker and potential treatment target in systemic lupus erythematosus. International Journal of Molecular Sciences, 25, 10845. https://doi.org/10.3390/ijms251910845
Sanges, S., Guerrier, T., Duhamel, A., Guilbert, L., Hauspie, C., Largy, A., Balden, M., Podevin, C., Lefèvre, G., Jendoubi, M., et al. (2022). Soluble markers of B cell activation suggest a role of B cells in the pathogenesis of systemic sclerosis-associated pulmonary arterial hypertension. Frontiers in Immunology, 13, 954007. https://doi.org/10.3389/fimmu.2022.954007
Food and Drug Administration. (2022, October 25). FDA approves teclistamab-cqyv for relapsed or refractory multiple myeloma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-teclistamab-cqyv-relapsed-or-refractory-multiple-myeloma
Food and Drug Administration. (2023, August 14). FDA grants accelerated approval to elranatamab-bcmm for multiple myeloma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-elranatamab-bcmm-multiple-myeloma
Food and Drug Administration. (2025, July 2). FDA grants accelerated approval to linvoseltamab-gcpt for relapsed or refractory multiple myeloma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-linvoseltamab-gcpt-relapsed-or-refractory-multiple-myeloma
Otsuka Pharmaceutical Co., Ltd. (2025, June 23). Otsuka obtains exclusive license from Harbour BioMed for HBM7020: A novel BCMA×CD3 bispecific T-cell engager. https://www.otsuka.co.jp/en/company/newsreleases/2025/20250623_1.html
ClinicalTrials.gov. (n.d.-a). Study of CM336 in Relapsed or Refractory Multiple Myeloma Patients (NCT07181239). https://clinicaltrials.gov/study/NCT07181239
ClinicalTrials.gov. (n.d.-b). GR1803 Injection in Patients With Relapsed/Refractory Multiple Myeloma (NCT06566547). https://clinicaltrials.gov/study/NCT06566547
ClinicalTrials.gov. (n.d.-c). A Ph1/2 Study of EMB-06 in Participants With Relapsed or Refractory Myeloma (NCT04735575). https://clinicaltrials.gov/study/NCT04735575
ClinicalTrials.gov. (n.d.-d). A Study of JNJ-79635322 in Participants With Relapsed or Refractory Multiple Myeloma or Previously Treated Amyloid Light-chain (AL) Amyloidosis (NCT05652335). https://clinicaltrials.gov/study/NCT05652335
ClinicalTrials.gov. (n.d.-e). Study of ISB 2001 in Relapsed/Refractory Multiple Myeloma (TRIgnite-1) (NCT05862012). https://clinicaltrials.gov/study/NCT05862012
Food and Drug Administration. (2025, October 22). FDA approves belantamab mafodotin blmf for relapsed or refractory multiple myeloma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belantamab-mafodotin-blmf-relapsed-or-refractory-multiple-myeloma