Autoimmune diseases represent the second largest category of chronic diseases worldwide. The patient population is large and the unmet medical need remains significant, driving rapid growth in the targeted therapy market. As the first class of targeted biologic agents, TNF-α inhibitors have become a core therapeutic category in autoimmune disease treatment due to their well-defined anti-inflammatory mechanisms and broad range of indications.
Recently, progress reported by Sorriso Pharmaceuticals has drawn renewed attention to this field. Its orally administered bispecific antibody SOR102 targets both TNF-α and IL-23p19 for the treatment of ulcerative colitis. Phase I clinical data demonstrated favorable safety and preliminary efficacy, with a 56% clinical response rate, and a Phase II study is planned. The advancement of emerging pipelines has refocused industry attention on the value of the well-established TNF-α target.
Overview of the TNF Target
Tumor necrosis factor (TNF) is an inflammatory cytokine that coordinates tissue homeostasis by regulating the production of other cytokines, as well as cell survival and cell death.
TNF exists in two forms: TNF-α and TNF-β. Although TNF-α and TNF-β share only approximately 30% sequence homology, they bind to the same receptors. TNF-α accounts for 70%–95% of the total biological activity of TNF; therefore, TNF generally refers to TNF-α in most contexts.
TNF-α (Tumor Necrosis Factor alpha) is a homotrimeric protein composed of 157 amino acids. It is primarily produced and secreted by activated macrophages, as well as other cell types such as CD4+ T cells, neutrophils, and mast cells. TNF-α can induce inflammatory responses and apoptosis and plays a critical role in immune responses, inflammatory processes, and the regulation of cell death.
TNF Signaling Pathway
The biological functions of TNF depend on its binding to receptors and the subsequent activation of downstream signaling pathways. The primary TNF receptors include TNFR1 (TNF Receptor 1) and TNFR2 (TNF Receptor 2), which differ significantly in structure, expression patterns, and regulatory functions.
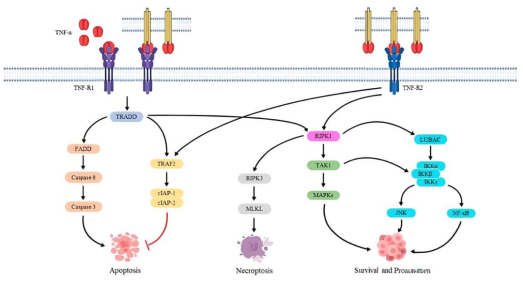
Among them, TNFR1 mainly mediates pro-inflammatory effects. When cells are exposed to inflammatory stimuli or stress, TNFR1 expression is upregulated. Binding of TNF-α to TNFR1 activates a series of signaling pathways, leading to biological processes such as apoptosis and inflammatory responses. Based on this mechanism, blocking the TNF–TNFR1 signaling axis by targeting TNFR1 and its ligand TNF has become an important therapeutic strategy for inflammatory diseases.
In contrast, TNFR2 primarily functions in immune regulation and tissue repair. It is highly expressed on immune cells such as regulatory T cells (Tregs) and on endothelial cells. Upon binding to TNF-α, TNFR2 preferentially activates the PI3K/Akt pathway, promoting cell survival, proliferation, immune tolerance, and tissue repair. Under conditions of chronic inflammation, the pro-inflammatory effects of TNFR2 can also be significantly enhanced, indicating that its function is context dependent.
Progress in the Development of TNF/TNFR-Targeted Therapies
At present, multiple TNF/TNFR-targeted drugs are under development. The therapeutic modalities include monoclonal antibodies, bispecific antibodies, ADCs, and fusion proteins. These candidates are primarily being developed for autoimmune diseases such as rheumatoid arthritis (RA) and severe psoriasis, among others.
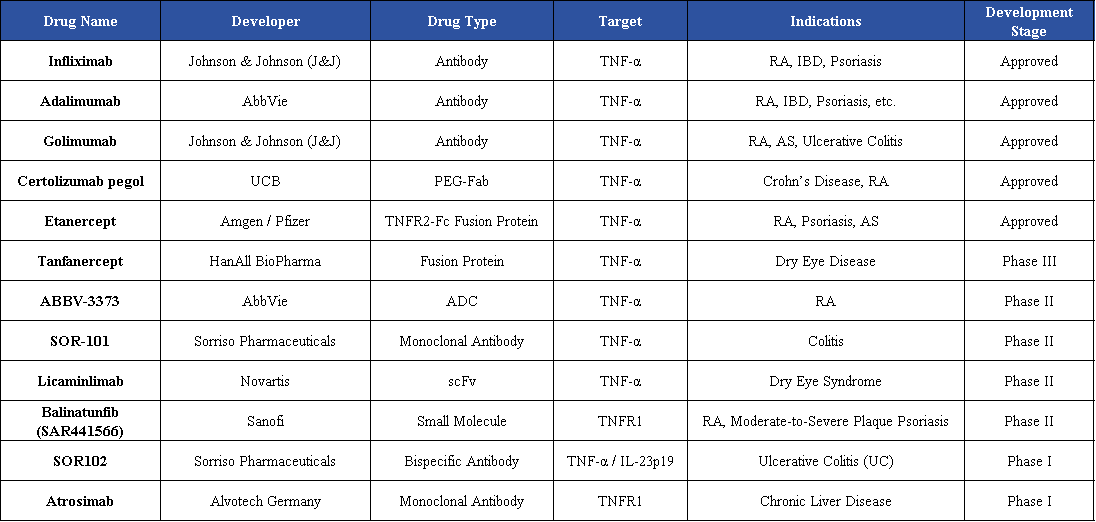
Sorriso Pharmaceuticals has established two innovative TNF-α–targeted programs in the field of oral antibody therapeutics, including the monoclonal antibody SOR-101 and the bispecific antibody SOR102, covering distinct mechanisms of action and indications.
SOR-101 is an orally administered anti–TNF-α monoclonal antibody (antibody fragment / Vorabody) developed by Sorriso Pharmaceuticals and is currently in Phase II clinical development. It is primarily being developed for inflammatory bowel diseases such as colitis. The molecule is designed to reach sites of intestinal inflammation following oral administration and to effectively bind TNF-α, thereby locally suppressing inflammatory responses. Unlike traditional injectable or infusion-based anti–TNF therapies, SOR-101 is intended to achieve localized inhibition of TNF-α in the gut, with the potential to improve safety and patient adherence.
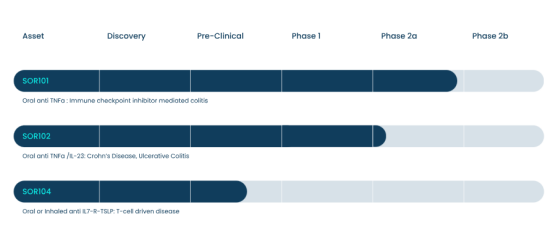
Through protein engineering and specialized formulation design, SOR102 achieves targeted release in the gastrointestinal tract and has become the first oral bispecific antibody drug to enter clinical development. Recently, its clinical results were published for the first time in The Lancet, demonstrating favorable efficacy.
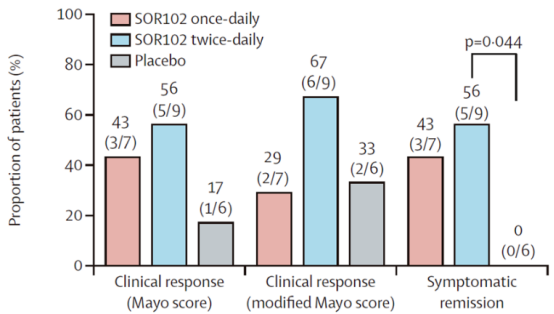
In Part 3 of the study, on Day 42, the clinical response rates based on the Mayo score were 43% (3/7) and 56% (5/9) in the once-daily and twice-daily SOR102 dosing groups, respectively. The modified Mayo response rates were 29% (2/7) and 67% (6/9), and the symptom remission rates were 43% (3/7) and 56% (5/9).
In contrast, the corresponding rates in the placebo group were only 17% (1/6), 33% (2/6), and 0%, respectively.
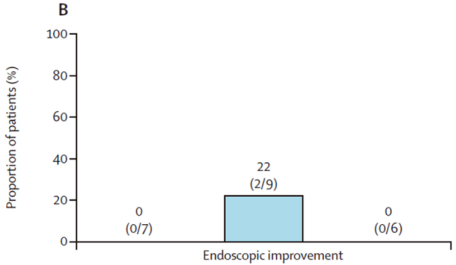
In addition, two patients (2/9) in the twice-daily dosing group achieved clinical remission accompanied by endoscopic improvement.
Preliminary clinical data demonstrate that this oral bispecific antibody not only shows early signs of efficacy but also exhibits a favorable safety profile. Sorriso Pharmaceuticals stated that a Phase II clinical trial is planned to be initiated later this year, with further efficacy data anticipated.
Beyond Sorriso, global development efforts targeting the TNF-α/TNFR pathway are advancing across multiple technological approaches. Companies such as Harbour BioMed and Novartis are developing fusion proteins and scFv-based therapeutics for dry eye disease, currently in Phase III and Phase II, respectively. Sanofi and AbbVie are advancing TNFR1-targeted small molecules and antibody–drug conjugates (ADCs) for indications including rheumatoid arthritis and psoriasis, both in Phase II development. Alvotech is also conducting a Phase I study of a TNFR1 monoclonal antibody. Overall, development strategies are shifting from conventional antibodies toward localized delivery, small-molecule modalities, and receptor-selective targeting.
Conclusion
Driven by the pursuit of more precise and more convenient therapeutic strategies, the innovative potential of the TNF-α/TNFR pathway continues to expand, positioning it as a key axis in reshaping the treatment landscape for autoimmune diseases.
Reference
U.S. Food and Drug Administration. (2021). Remicade (infliximab) injection: Prescribing information (BLA 103772). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103772s5401lbl.pdf
U.S. Food and Drug Administration. (2018). Humira (adalimumab) injection, for subcutaneous use: Prescribing information (BLA 125057). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125057s410lbl.pdf
Johnson & Johnson. (2018, September 7). U.S. FDA approves Simponi (golimumab) for the treatment of pediatric ulcerative colitis. https://www.jnj.com/media-center/press-releases/u-s-fda-approves-simponi-golimumab-for-the-treatment-of-pediatric-ulcerative-colitis
U.S. Food and Drug Administration. (2008). Enbrel (etanercept): Biologics license application approval package (BLA 125160). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/125160s000TOC2.cfm
Amgen Inc. (2004, April 30). FDA approves Enbrel to treat psoriasis; new convenient treatment provides rapid and significant relief of symptoms. https://www.amgen.com/newsroom/press-releases/2004/04/fda-approves-enbrel-to-treat-psoriasis-new-convenient-treatment-provides-rapid-and-significant-relief-of-symptoms
ClinicalTrials.gov. (2022). A study to evaluate [study title unavailable from URL] (Identifier: NCT05109702). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT05109702
AbbVie Inc. (2020, June 10). Novel antibody-drug conjugate ABBV-3373 shows improvement in disease activity in Phase 2a study of patients with rheumatoid arthritis. https://news.abbvie.com/2020-06-10-Novel-Antibody-Drug-Conjugate-ABBV-3373-Shows-Improvement-in-Disease-Activity-in-Phase-2a-Study-of-Patients-with-Rheumatoid-Arthritis
Sorriso Pharmaceuticals. (n.d.). SOR101: Oral anti-TNFα VORABODY™. https://sorrisopharma.com/science/sor101-oral-anti-tnfa-vorabody/
ClinicalTrials.gov. (2023). A study to evaluate [study title unavailable from URL] (Identifier: NCT05896670). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT05896670
ClinicalTrials.gov. (2024). A study to evaluate [study title unavailable from URL] (Identifier: NCT07222189). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT07222189
ClinicalTrials.gov. (2023). A study to evaluate [study title unavailable from URL] (Identifier: NCT06080048). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT06080048
ClinicalTrials.gov. (2021). A study to evaluate [study title unavailable from URL] (Identifier: NCT04650126). U.S. National Library of Medicine. https://clinicaltrials.gov/study/NCT04650126