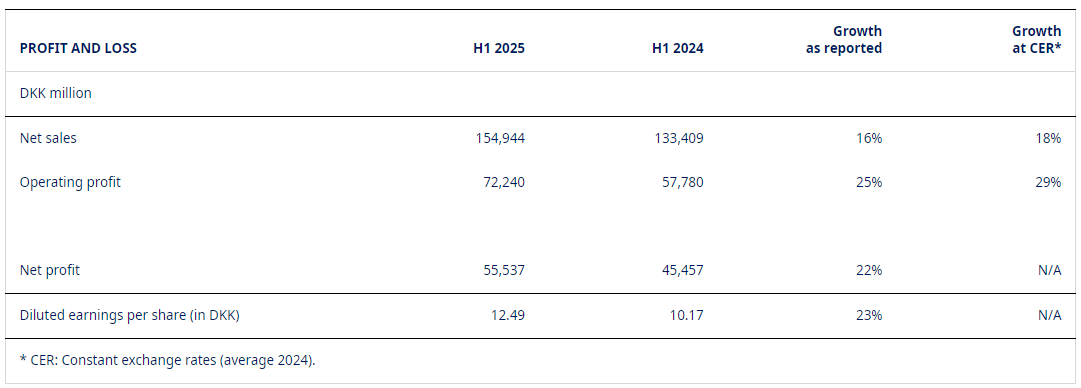
In the first six months of 2025, Novo Nordisk delivered robust financial performance, underpinned by exceptional growth in its GLP-1-based treatments for diabetes and obesity. Total sales increased by 16% in Danish kroner (18% at constant exchange rates), reaching DKK 154.9 billion, while operating profit rose by 25% in Danish kroner (29% at CER) to DKK 72.2 billion.
The company's Diabetes and Obesity care segment was the primary growth engine, generating DKK 145.4 billion in sales, up 16% in DKK (18% at CER). This strong performance was mainly driven by explosive growth in Obesity care, where sales surged by 56% in DKK (58% at CER) to DKK 38.8 billion. Notably, GLP-1 diabetes treatments grew by 8% in DKK (10% at CER), reflecting continued demand and leadership in this category. Learn more about our GLP-1R/GCGR/GIPR catalog.
Growth in the U.S. market remained solid with sales up 16% in DKK (17% at CER), supported by gross-to-net adjustments, including a one-time DKK 3 billion impact from the 340B provision. International Operations also showed resilience, with sales rising 16% in DKK (19% at CER).
Within R&D, Novo Nordisk is advancing its GLP-1 pipeline with confidence. Amycretin, both subcutaneous and oral formulations, will move into phase 3 trials for weight management. Meanwhile, the REDEFINE 11 trial has been initiated to further explore the efficacy and safety of CagriSema, and a higher dose of semaglutide (7.2 mg) has been submitted for regulatory approval in the EU.
Despite the strong first-half performance, Novo Nordisk has revised its full-year 2025 outlook due to headwinds in the second half, including continued use of compounded GLP-1s, slower-than-expected market expansion, and increased competition. Sales growth is now expected at 8-14% CER, with operating profit expected to grow 10-16% CER. However, reported growth in Danish kroner is forecast to be 3 and 5 percentage points lower, respectively.
The outlook revision reflects lower-than-anticipated growth for Wegovy® in the U.S. obesity market, Ozempic® in the U.S. diabetes market, and Wegovy® in certain international markets. Nevertheless, the company remains committed to the global rollout of Wegovy® and is ramping up commercial efforts to drive further market penetration for both Wegovy® and Ozempic®.
Leadership changes were also announced, with Maziar Mike Doustdar succeeding Lars Fruergaard Jørgensen as President and CEO, effective August 7, alongside broader organizational restructuring to align R&D under Martin Holst Lange.
Lars Fruergaard Jørgensen commented:
"While delivering 18% sales growth in the first half of 2025, we have lowered our full-year outlook due to lower growth expectations for our GLP-1 treatments in the second half. We are taking measures to sharpen our commercial execution and ensure efficiencies in our cost base while continuing to invest in future growth. With more than one billion people living with obesity globally, and only a few million on treatment, I am confident that under Mike Doustdar's leadership, Novo Nordisk will maximize the significant growth opportunities ahead."
With a market-leading GLP-1 portfolio, a robust pipeline, and continued investment in commercial scale-up, Novo Nordisk remains well-positioned to lead the global fight against diabetes and obesity in the years ahead.