On December 16, GSK announced that depemokimab (brand name: Exdensur) received FDA approval for the treatment of severe asthma. Just one day earlier, the drug was approved by the UK Medicines and Healthcare products Regulatory Agency (MHRA) for two indications: asthma and chronic rhinosinusitis with nasal polyps (CRSwNP).
As a long-acting biologic with a dosing interval that can be extended to once every six months, depemokimab represents an important advancement in the management of chronic diseases. It has also been identified by Evaluate as a “potential blockbuster” drug for 2025.
In immunology, cytokines are key molecules that regulate immune responses. Among them, interleukin-5 (IL-5) has attracted significant attention due to its critical role in the development and activation of eosinophils. The success of depemokimab validates the feasibility of long-term IL-5 inhibition strategies and provides an important direction for future development of this target across multiple indications and in combination therapies.
Decoding Immune Signaling: IL-5
Interleukin-5 (IL-5) is a Th2-derived homodimeric cytokine primarily secreted by T cells. It plays a crucial role in promoting the growth and differentiation of B cells and eosinophils.
The interleukin-5 receptor (IL-5R) belongs to the type I cytokine receptor family and consists of an IL-5–specific α subunit and a common βc subunit. The α subunit binds IL-5 and confers cytokine specificity to the receptor, while the β subunit contains the signal transduction domain and does not directly bind IL-5.
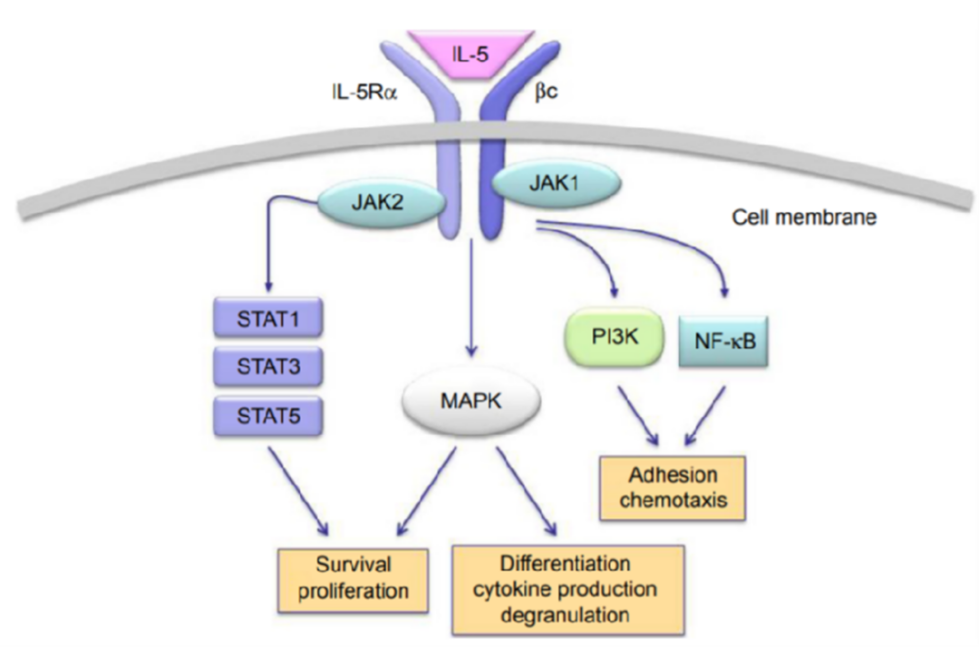
IL-5 activates the JAK–STAT signaling pathway by binding to its receptor IL-5Rα, leading to the phosphorylation and nuclear translocation of STAT1, STAT3, and STAT5, where they regulate the expression of target genes. Aberrant activation of this pathway drives a range of allergic and inflammatory diseases, including asthma, atopic dermatitis, chronic obstructive pulmonary disease (COPD), eosinophilic gastrointestinal disorders, hypereosinophilic syndrome, Churg–Strauss syndrome, and eosinophilic nasal polyps.
Although glucocorticoids remain the mainstay of treatment for these conditions, therapeutic responses are limited in some patients, and long-term use is associated with significant adverse effects.
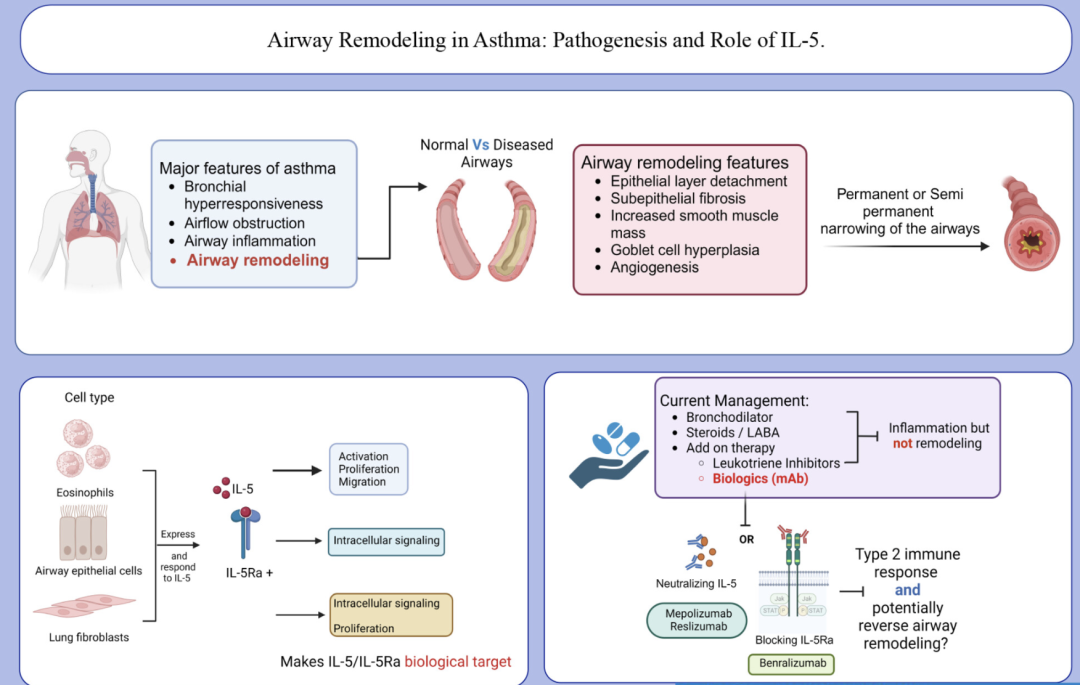
Drugs targeting IL-5 or its receptor can specifically block this signaling pathway, inhibiting the proliferation and activation of eosinophils. These therapies can significantly reduce the number of eosinophils in the blood and alleviate disease symptoms associated with their activity. In addition, they can improve airway inflammation and respiratory function, thereby enhancing patients’ quality of life.
Drug Development and Clinical Landscape Targeting IL-5
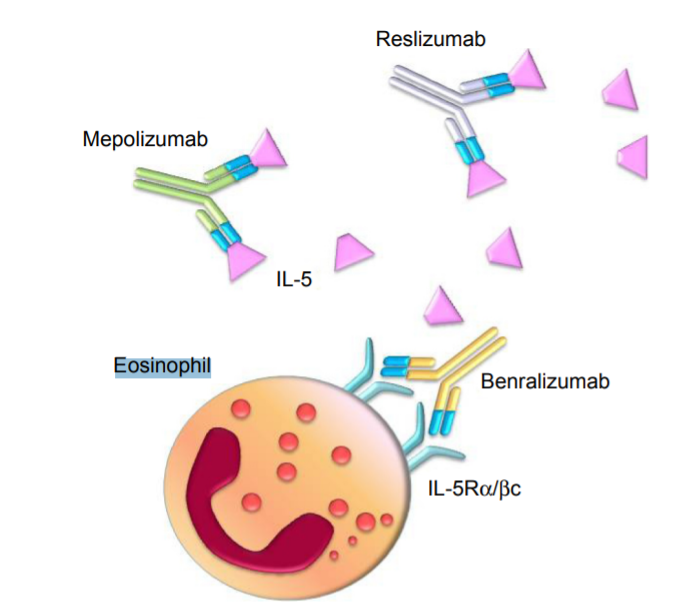
Antibody Therapeutics: Several monoclonal antibodies targeting IL-5 are currently under clinical development. These antibodies specifically bind to IL-5, blocking its interaction with the IL-5 receptor and thereby inhibiting the activation and proliferation of eosinophils.
Small-Molecule Therapeutics: In addition to antibody drugs, several small-molecule compounds have been developed to target IL-5, including synthetic IL-5Rα antagonists and IL-5Rα–specific inhibitors. These agents block the signaling pathway by binding to IL-5 or its receptor α subunit, suppressing eosinophil proliferation and activation. Some of these small-molecule drugs have already entered clinical trials.