In solid tumor research, tumor-adjacent tissues have long been considered largely passive structural components. Accumulating evidence now indicates that the tumor-adjacent microenvironment plays an active and regulatory role in tumor progression, rather than merely serving as a supportive backdrop.
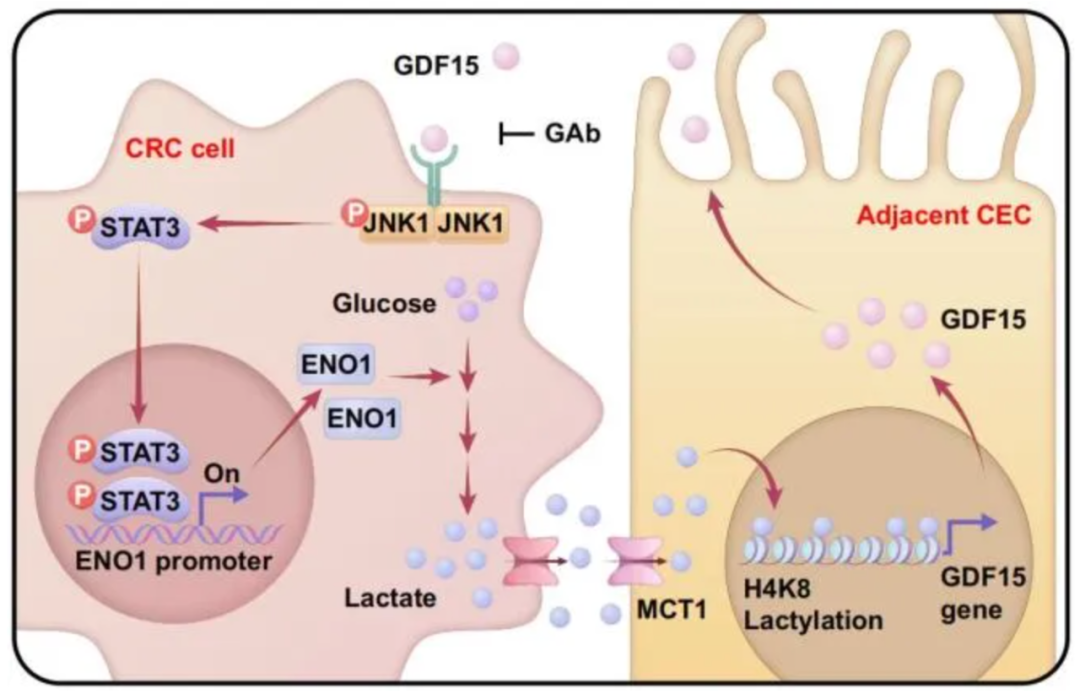
In a study published in Nature Aging (IF = 19.4) on December 3, 2025, researchers identified a previously unrecognized mechanism in which senescent tumor-adjacent colonic epithelial cells secrete GDF15, thereby modulating colorectal cancer progression through activation of a glycolysis–histone lactylation feedback loop.
The study uncovers a previously unrecognized metabolic–epigenetic communication axis between colorectal cancer cells and tumor-adjacent epithelial cells. By extending the functional scope of GDF15 beyond tumor cells themselves to include senescent tumor-adjacent epithelial cells and microenvironmental regulation, these findings substantially broaden the potential therapeutic landscape of GDF15 in oncology.
GDF15
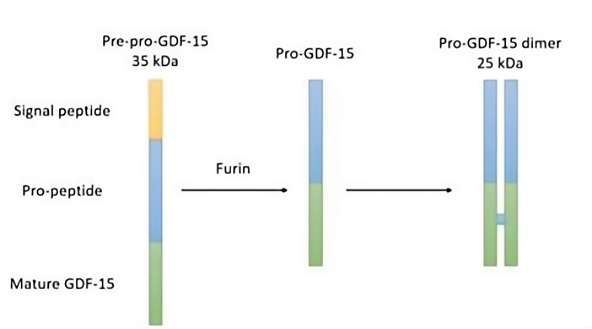
GDF15, also known as MIC-1, is a stress-induced secreted protein that circulates as a 25 kDa homodimer composed of two peptide chains, each containing 112 amino acids, linked by disulfide bonds. Under normal physiological conditions, GDF15 is expressed at low levels, with expression primarily upregulated in organs such as the liver, kidneys, heart, and lungs in response to injury or cellular stress.
Recent studies have found that GDF15 plays a key role in physiological and pathological processes such as inflammation regulation, tissue repair, metabolic disorders, and cellular stress. Its expression can be induced and upregulated by various stress factors, including cellular injury, oxidative stress, and antidiabetic drugs like metformin.
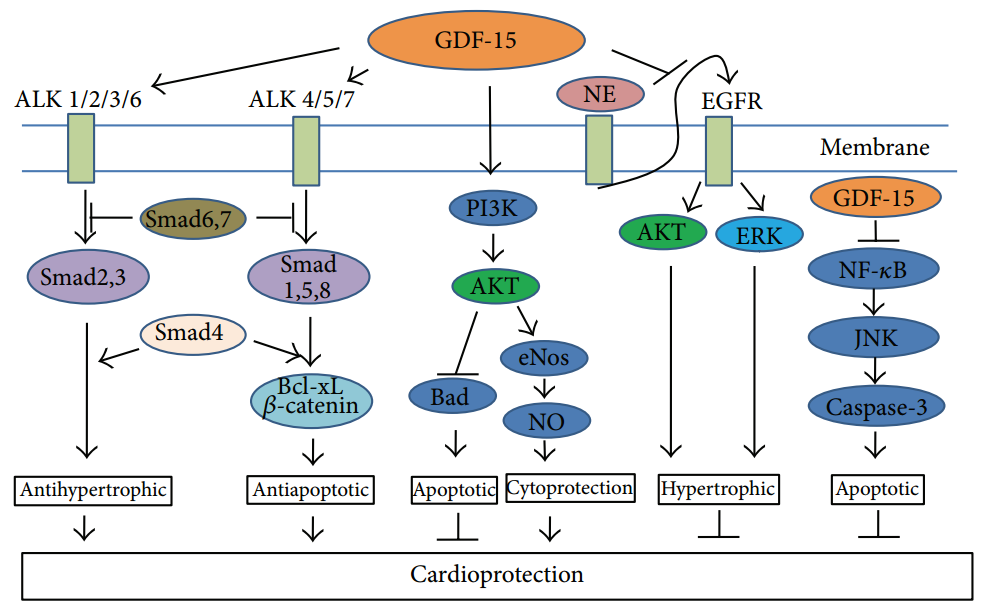
GDF-15 Mediated Signaling Pathway
GDF15 exerts its function by binding to its only known receptor, GFRAL, forming a ligand–receptor complex that recruits the co-receptor RET. This interaction activates a cascade of downstream signaling pathways, including RET, AKT, ERK, and PLC-γ1. GFRAL expression is restricted to the nucleus of the solitary tract in the medulla oblongata, which underlies the GDF15–GFRAL pathway’s central role in regulating appetite, energy metabolism, and body weight. This pathway has also shown potential therapeutic value in metabolic diseases such as cardiovascular disorders and non-alcoholic fatty liver disease.
Multi-Disease Therapeutic Potential of a Single Target
Tumor Immune Evasion: A New Target for Overcoming Immune Resistance
GDF15 is highly expressed in the tumor microenvironment, where it suppresses dendritic cell maturation, T cell activation, and infiltration, thereby helping tumors evade immune surveillance. Tumor cells exploit this mechanism, leading to resistance to immunotherapy. Targeting GDF15 holds promise for reversing immune suppression, enhancing the efficacy of PD-1/PD-L1 therapies, and offering a new treatment approach for patients with cold tumors.
Cancer Cachexia: A Potential Target to Improve Nutritional and Functional Status
GDF15 is one of the driving factors of cancer cachexia, a metabolic syndrome occurring in 50% to 80% of cancer patients. It is characterized by anorexia, rapid weight loss, and wasting of muscle and fat tissue, severely impacting quality of life and treatment tolerance. Currently, targeted antibodies against GDF15, such as Ponsegromab, are under development and have shown preliminary efficacy in improving body weight and physical function, offering a new approach for supportive cancer care.
Obesity and Diabetes: A Central Weight-Loss Target Following GLP-1
Around 2017, the medical community began recognizing the potential of GDF15 in obesity treatment. Pharmaceutical giants Eli Lilly, Novo Nordisk, and Johnson & Johnson simultaneously published findings in Nature Medicine, identifying GFRAL as the high-affinity receptor for GDF15 and a central player in appetite suppression and weight reduction. This marked the first time scientists had uncovered the core mechanism and key receptor by which GDF15 regulates body weight, offering a promising therapeutic target for obesity and type 2 diabetes.
On June 28, 2023, a research team led by Dr. Dongdong Wang from Dr. Gregory Steinberg’s lab at McMaster University in Canada published a study in Nature, systematically elucidating the mechanism by which GDF15 promotes weight loss by increasing energy expenditure in muscle.
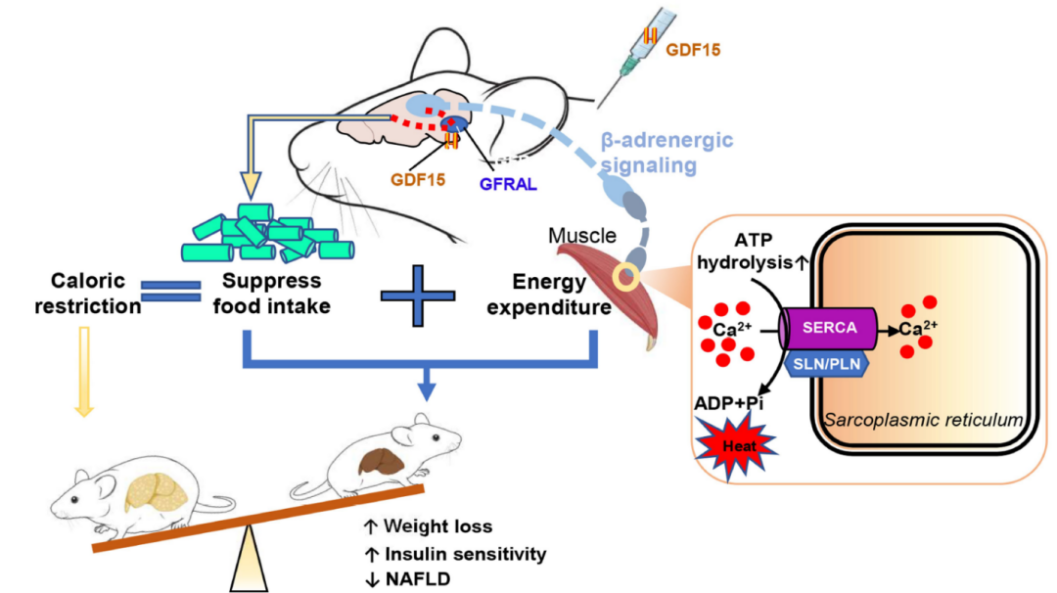
NAFLD and Cardiovascular Disease: A New Window into Inflammation and Metabolic Regulation
GDF15 can improve liver metabolism and reduce inflammation in non-alcoholic fatty liver disease (NAFLD). At the same time, as a stress-responsive biomarker for cardiovascular events, it holds potential for early diagnosis and intervention, emerging as a new focus in chronic disease management.
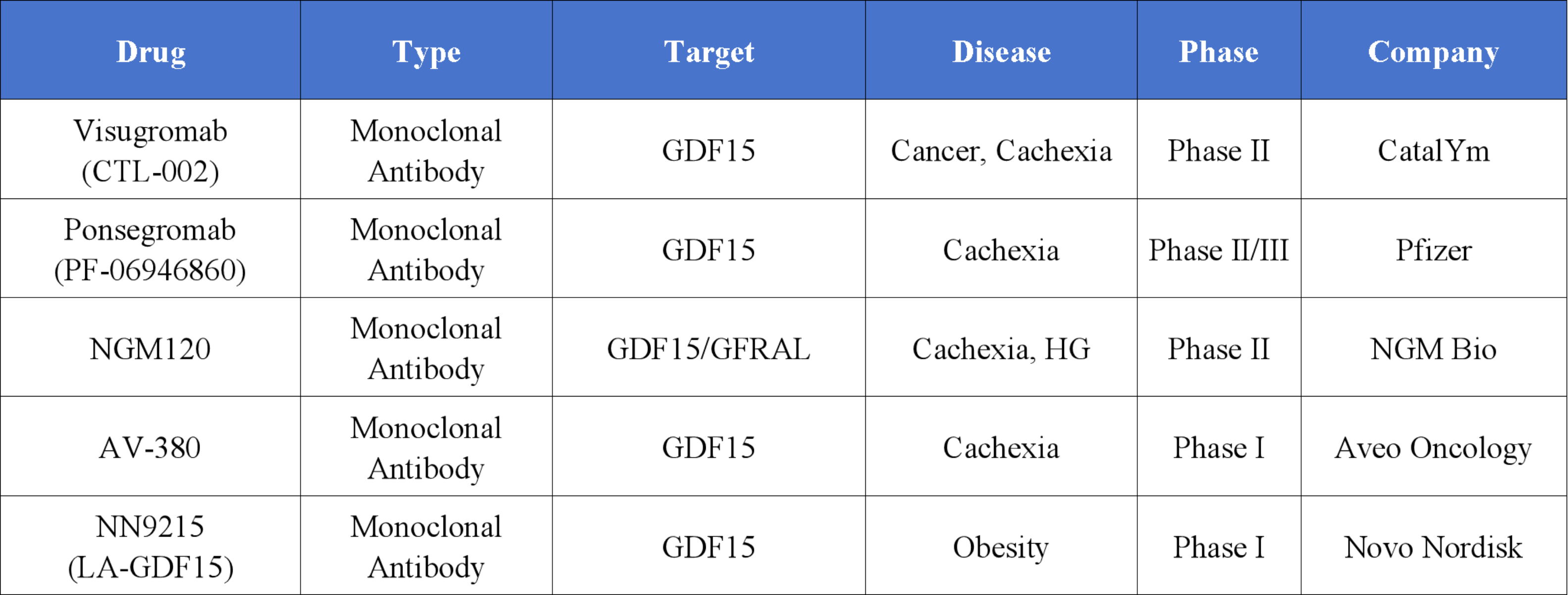
The Current Pipeline and Research Landscape Targeting GDF15
Globally, the development of GDF15-targeted therapies is still in the early stages. The majority of drug candidates are monoclonal antibodies, with the most advanced currently in Phase II clinical trials. Notable examples at this stage include Visugromab by CatalYm, Ponsegromab by Pfizer, and NGM120 by NGM Bio.
Anti-GDF15 Monoclonal Antibody of Pfizer: Ponsegromab
In September last year, Pfizer presented positive results from its Phase II clinical trial of Ponsegromab at the European Society for Medical Oncology (ESMO) Annual Congress. Across all tested doses, the Ponsegromab-treated groups showed weight increases from baseline. At the highest dose assessed at week 12, patients experienced an average weight gain of 5.6% compared to the placebo group. Ponsegromab was considered safe and well-tolerated at all dose levels, with improvements observed in appetite, cachexia-related symptoms, physical activity, and muscle mass at the highest dose tested.
Anti-GDF15 Monoclonal Antibody of CatalYm: Visugromab
Visugromab is a locally acting immunomodulator that neutralizes tumor-derived GDF-15 and is designed to overcome resistance to immunotherapy. At the 2024 ASCO Annual Meeting, results from a Phase I clinical study were presented, showing that neutralizing GDF-15 with Visugromab can reverse key mechanisms of cancer resistance. It restores effective anti-tumor responses by reactivating immune cells and promoting tumor infiltration.
Visugromab demonstrated good safety in Phase I trials and is currently undergoing Phase II clinical studies targeting multiple solid tumor indications.
Summary
Overall, GDF15 is gradually evolving from a stress- and metabolism-related factor into a key hub linking the tumor microenvironment, immune regulation, and systemic energy metabolism. Recent findings in Nature Aging indicate that GDF15 derived from senescent tumor-adjacent epithelial cells can continuously drive tumor progression through metabolic–epigenetic mechanisms, significantly expanding the functional scope of this target in solid tumors.
Considering its ongoing clinical development in cancer cachexia, immune resistance, and metabolic diseases, GDF15 demonstrates clear multi-disease therapeutic potential and holds promise as a strategic entry point for interventions that integrate immunity, metabolism, and tumor microenvironment modulation.
Reference
Adela, R., & Banerjee, S. K. (2015). GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: A translational prospective. Journal of Diabetes Research, 2015, 1–14. https://doi.org/10.1155/2015/490842
Aveo Oncology. (n.d.). AV-380 (Anti-GDF15 IGG1 [MAB]) in cancer cachexia. https://www.aveooncology.com/our-science/av-380/
Catalym. (n.d.). Pipeline. https://www.catalym.com/pipeline/
Delrue, C., Speeckaert, R., Delanghe, J. R., & Speeckaert, M. M. (2023). Growth differentiation factor 15 (GDF-15) in kidney diseases. Advances in Clinical Chemistry, 114, 1–46. https://doi.org/10.1016/bs.acc.2023.02.003
Dufour, I., Bindels, L. B., & Goffin, E. (2025). Ponsegromab for the treatment of cancer cachexia. New England Journal of Medicine, 392(10), 1035–1037. https://doi.org/10.1056/nejmc2500502
Emmerson, P. J., Wang, F., Du, Y., Liu, Q., Pickard, R. T., Gonciarz, M. D., Coskun, T., Hamang, M. J., Sindelar, D. K., Ballman, K. K., Foltz, L. A., Muppidi, A., Alsina-Fernandez, J., Barnard, G. C., Tang, J. X., Liu, X., Mao, X., Siegel, R., Sloan, J. H., … Wu, X. (2017). The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nature Medicine, 23(10), 1215–1219. https://doi.org/10.1038/nm.4393
ESMO. (2024). Inhibition of GDF-15 with Ponsegromab results in increased weight gain and overall activity level and reduced cancer cachexia symptoms. European Society for Medical Oncology. https://www.esmo.org/oncology-news/inhibition-of-gdf-15-with-ponsegromab-results-in-increased-weight-gain-and-overall-activity-level-and-reduced-cancer-cachexia-symptoms
Mullican, S. E., Lin-Schmidt, X., Chin, C. N., Chavez, J. A., Furman, J. L., Armstrong, A. A., Beck, S. C., South, V. J., Dinh, T. Q., Cash-Mason, T. D., Cavanaugh, C. R., Nelson, S., Huang, C., Hunter, M. J., & Rangwala, S. M. (2017). GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nature Medicine, 23(10), 1150–1157. https://doi.org/10.1038/nm.4392
NGM Biopharmaceuticals. (2025). A potential treatment for pregnant women with hyperemesis gravidarum. https://www.ngmbio.com/pipeline/ngm120/
Pfizer. (2024). Pfizer presents positive data from phase 2 study of Ponsegromab in patients with cancer cachexia. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-presents-positive-data-phase-2-study-ponsegromab
Wang, D., Townsend, L. K., DesOrmeaux, G. J., et al. (2023). GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature, 619, 143–150. https://doi.org/10.1038/s41586-023-06249-4
Yang, L., Chang, C., Sun, Z., Madsen, D., Zhu, H., Padkjær, S. B., Wu, X., Huang, T., Hultman, K., Paulsen, S. J., Wang, J., Bugge, A., Frantzen, J. B., Nørgaard, P., Jeppesen, J. F., Yang, Z., Secher, A., Chen, H., Li, X., … Jørgensen, S. B. (2017). GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nature Medicine, 23(10), 1158–1166. https://doi.org/10.1038/nm.4394
ASCO. (2024). Effects of neutralization of tumor-derived immunosuppressant GDF-15 on anti-PD-1 activity in anti-PD-(L)1 relapsed/refractory non-squamous NSCLC, urothelial, and hepatocellular cancer. https://www.asco.org/abstracts-presentations/ABSTRACT446680
Nature Aging. (2025). Growth differentiation factor 15 secreted by senescent tumor-adjacent colonic epithelial cells drives colorectal cancer progression. https://www.nature.com/articles/s43587-025-01023-9