On December 16, GSK announced that depemokimab (brand name: Exdensur) received FDA approval for the treatment of severe asthma. Just one day earlier, the drug was approved by the UK Medicines and Healthcare products Regulatory Agency (MHRA) for two indications: asthma and chronic rhinosinusitis with nasal polyps (CRSwNP).
As a long-acting biologic with a dosing interval that can be extended to once every six months, depemokimab represents an important advancement in the management of chronic diseases. It has also been identified by Evaluate as a “potential blockbuster” drug for 2025.
In immunology, cytokines are key molecules that regulate immune responses. Among them, interleukin-5 (IL-5) has attracted significant attention due to its critical role in the development and activation of eosinophils. The success of depemokimab validates the feasibility of long-term IL-5 inhibition strategies and provides an important direction for future development of this target across multiple indications and in combination therapies.
Decoding Immune Signaling: IL-5
Interleukin-5 (IL-5) is a Th2-derived homodimeric cytokine primarily secreted by T cells. It plays a crucial role in promoting the growth and differentiation of B cells and eosinophils.
The interleukin-5 receptor (IL-5R) belongs to the type I cytokine receptor family and consists of an IL-5–specific α subunit and a common βc subunit. The α subunit binds IL-5 and confers cytokine specificity to the receptor, while the β subunit contains the signal transduction domain and does not directly bind IL-5.
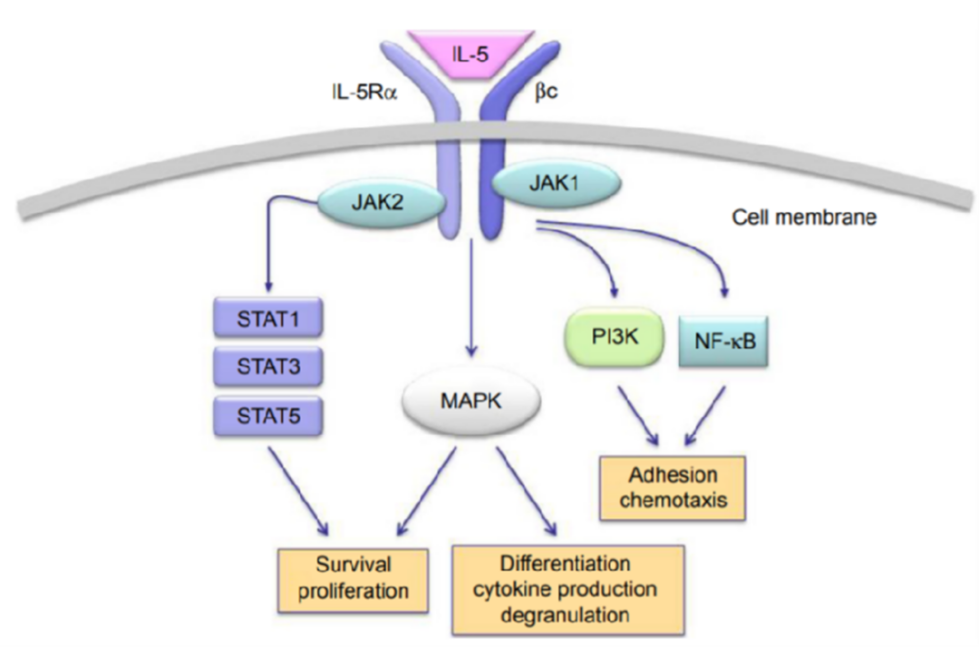
IL-5 activates the JAK–STAT signaling pathway by binding to its receptor IL-5Rα, leading to the phosphorylation and nuclear translocation of STAT1, STAT3, and STAT5, where they regulate the expression of target genes. Aberrant activation of this pathway drives a range of allergic and inflammatory diseases, including asthma, atopic dermatitis, chronic obstructive pulmonary disease (COPD), eosinophilic gastrointestinal disorders, hypereosinophilic syndrome, Churg–Strauss syndrome, and eosinophilic nasal polyps.
Although glucocorticoids remain the mainstay of treatment for these conditions, therapeutic responses are limited in some patients, and long-term use is associated with significant adverse effects.
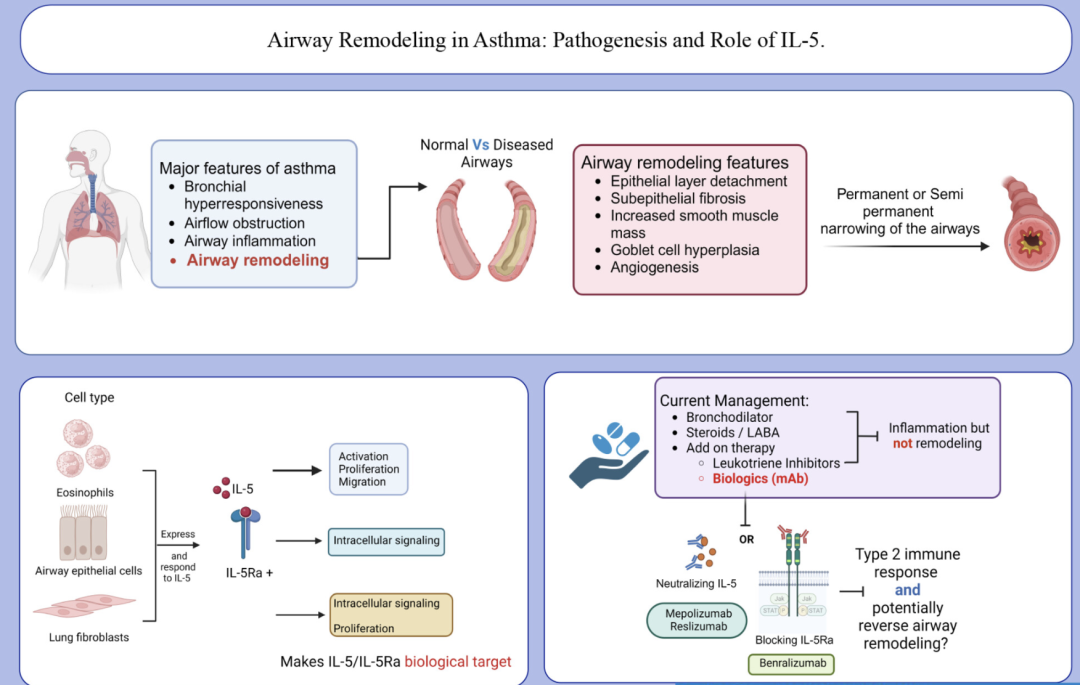
Drugs targeting IL-5 or its receptor can specifically block this signaling pathway, inhibiting the proliferation and activation of eosinophils. These therapies can significantly reduce the number of eosinophils in the blood and alleviate disease symptoms associated with their activity. In addition, they can improve airway inflammation and respiratory function, thereby enhancing patients’ quality of life.
Drug Development and Clinical Landscape Targeting IL-5
Antibody Therapeutics: Several monoclonal antibodies targeting IL-5 are currently under clinical development. These antibodies specifically bind to IL-5, blocking its interaction with the IL-5 receptor and thereby inhibiting the activation and proliferation of eosinophils.
Small-Molecule Therapeutics: In addition to antibody drugs, several small-molecule compounds have been developed to target IL-5, including synthetic IL-5Rα antagonists and IL-5Rα–specific inhibitors. These agents block the signaling pathway by binding to IL-5 or its receptor α subunit, suppressing eosinophil proliferation and activation. Some of these small-molecule drugs have already entered clinical trials.
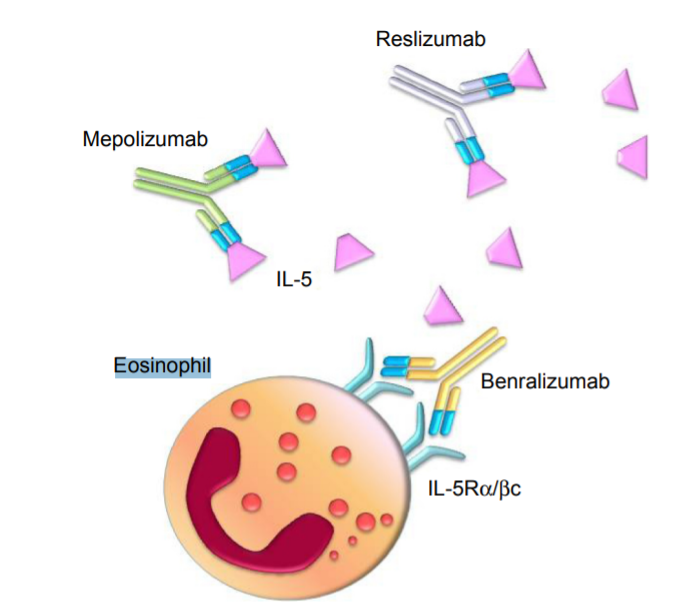
Based on their targets, IL-5–targeting drugs can be broadly classified into two categories: those targeting IL-5 and those targeting IL-5Rα. Currently, four IL-5–targeting drugs have been approved worldwide for the treatment of asthma and other eosinophil-related diseases. These include three IL-5 antibodies—Reslizumab, Mepolizumab, and Depemokimab—and one IL-5Rα antibody—Benralizumab.
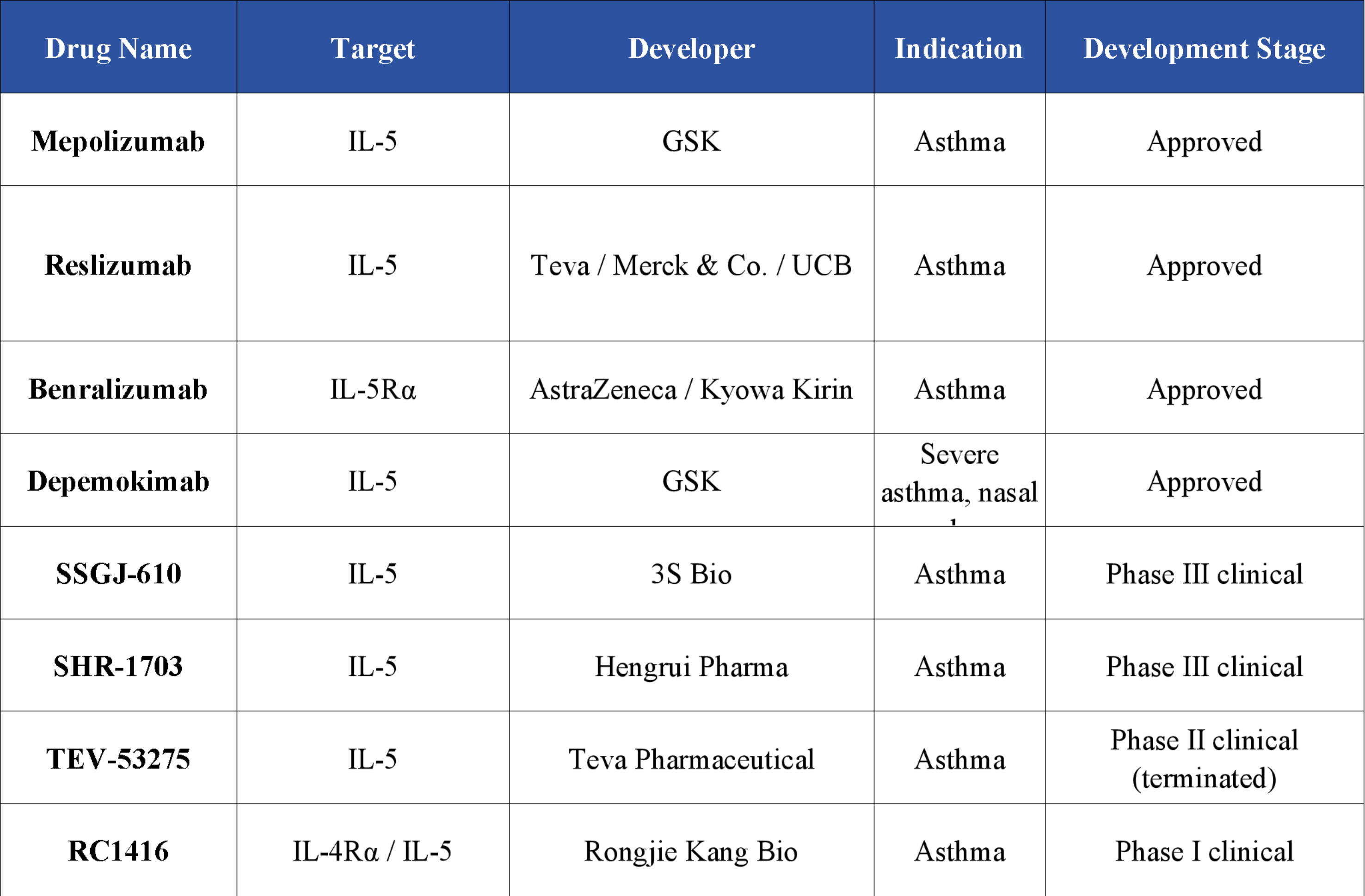
Mepolizumab (Nucala), a flagship product of GSK, was approved in the United States in November 2015 as the world’s first IL-5 monoclonal antibody. Since its launch, sales have steadily grown, reaching $2.279 billion globally in 2024, representing a 10% year-on-year increase.
Nucala has also received FDA approval for chronic obstructive pulmonary disease (COPD) with an eosinophilic phenotype. In China, it is approved for eosinophilic granulomatosis with polyangiitis (EGPA), severe eosinophilic asthma (SEA), and chronic rhinosinusitis with nasal polyps (CRSwNP), and has been successfully included in the national reimbursement drug list. With patents in major markets approaching expiration, the drug is expected to face competition from biosimilars in the future.
GSK’s Next-Generation Ultra-Long-Acting IL-5 Antibody: Depemokimab (Exdensur)
To address patent expiration risks and improve patient adherence, GSK developed Depemokimab, a next-generation ultra-long-acting IL-5 antibody. By optimizing the Fc region or heavy-chain sequence, the drug enhances interactions with the FcRn receptor or delays clearance, significantly extending its circulating half-life and allowing dosing every six months. Recently, Depemokimab received FDA approval for the treatment of severe eosinophilic asthma in adults and adolescents aged 12 years and older, making it the world’s first approved ultra-long-acting IL-5 antibody.
The asthma indication approval was based on the SWIFT-1 and SWIFT-2 studies, which showed that patients receiving Depemokimab twice per year had annualized asthma exacerbation rates reduced by 58% and 48% over 52 weeks compared with placebo. Approval for chronic rhinosinusitis with nasal polyps (CRSwNP) was supported by the ANCHOR study, demonstrating significant improvements in endoscopic total nasal polyp score and nasal obstruction score over 52 weeks. The drug was well tolerated, with adverse event rates and severity comparable to placebo, consolidating GSK’s market position in respiratory diseases and representing a potential future blockbuster.
In addition to GSK’s Mepolizumab and Depemokimab, Reslizumab and Benralizumab have also been approved globally, targeting IL-5 and IL-5Rα, respectively, for patients with severe eosinophilic asthma. Beyond these approved drugs, multiple IL-5–targeting candidates are at various stages of clinical development, offering potential new treatment options for eosinophil-related diseases.
Conclusion
IL-5 plays a central role in the development and activation of eosinophils, driving a range of allergic and inflammatory diseases. Targeting IL-5 or its receptor has proven to be an effective therapeutic strategy, as evidenced by the clinical success of monoclonal antibodies such as Mepolizumab, Depemokimab, Reslizumab, and Benralizumab. The approval of next-generation long-acting agents like Depemokimab highlights ongoing innovations aimed at improving patient adherence and outcomes. With multiple IL-5–targeting therapies already on the market and others in development, the IL-5 pathway remains a promising avenue for treating eosinophil-related diseases and addressing unmet medical needs in severe asthma, CRSwNP, and other conditions.
Reference
GSK. (2025). Nucala (mepolizumab) approved by US FDA for use in adults with chronic obstructive pulmonary disease (COPD). GSK. https://www.gsk.com/en-gb/media/press-releases/nucala-mepolizumab-approved-by-us-fda/
Teva USA. (n.d.). Teva announces FDA approval of CINQAIR® (reslizumab) injection. Teva. https://www.tevapharm.com/news-and-media/latest-news/teva-announces-fda-approval-of-cinqair-reslizumab-injection/
FASENRA. (n.d.). Severe eosinophilic asthma treatment | FASENRA® (benralizumab) subcutaneous injection. https://www.fasenra.com/
GSK. (2025). Exdensur (depemokimab) approved by US FDA for the treatment of severe asthma. GSK. https://www.gsk.com/en-gb/media/press-releases/exdensur-depemokimab-approved-by-us-fda-for-the-treatment-of-severe-asthma/
National Library of Medicine. (n.d.). Efficacy and safety study of 610 in patients with severe asthma (ClinicalTrials.gov identifier NCT06323213). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT06323213
National Library of Medicine. (n.d.). A study to evaluate the efficacy and safety of SHR‑1703 in subjects with eosinophilic granulomatosis with polyangiitis (ClinicalTrials.gov identifier NCT05979051). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT05979051
Teva Branded Pharmaceutical Products, R&D Inc. (n.d.). A study to test if TEV‑53275 is effective in relieving asthma (ClinicalTrials.gov identifier NCT04847674). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04847674
National Library of Medicine. (n.d.). Clinical trial record for NCT06067490. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT06067490