At the start of 2026, the B7-H3 ADC field received significant attention.
DualityBio announced an exclusive licensing collaboration with Roche for the B7-H3 ADC drug YL201. Under the agreement, both parties will jointly advance the development and commercialization of this B7-H3–targeting ADC across multiple solid tumor indications. The substantial upfront payment has quickly drawn renewed interest to the B7-H3 target.
On January 19, InnoLake licensed its B7-H3 ADC to the UK-based Ellipses Pharma, marking another international collaboration.
These two deals, completed within less than two weeks, highlight the market value of B7-H3 as a target in tumor immunotherapy and have brought the field back to industry focus.
In recent years, with continuous accumulation of clinical data and an increase in deal activity, the commercialization landscape for B7-H3 ADCs is accelerating. From early-stage exploration to multinational companies taking the lead, this path is gradually defining the potential of the next major ADC development.
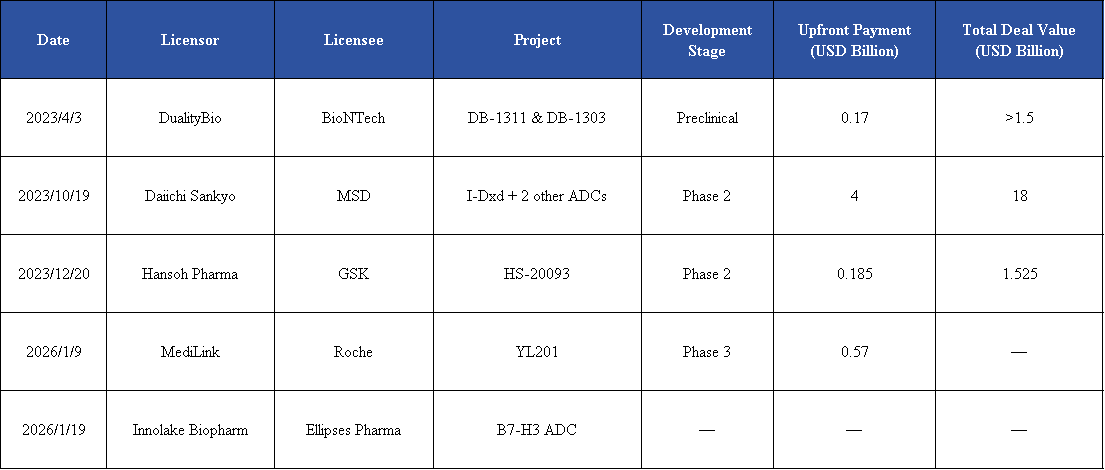
Overview of B7-H3 ADC BD transactions in recent years
Introduction to B7-H3 (CD276)
B7-H3, also known as CD276, is a type I transmembrane glycoprotein and a member of the B7 family. It was first identified in 2001.
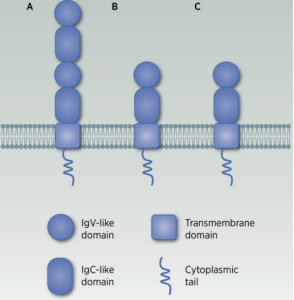
The B7H3 gene is located on chromosome 15 and encodes a protein of 316 amino acids with a relative molecular weight of 45–66 kDa. The full B7-H3 protein consists of four regions: a predicted signal peptide, extracellular V-like and C-like Ig domains (IgV and IgC), a transmembrane domain, and a cytoplasmic tail. There are two isoforms: 2Ig B7-H3 and 4Ig B7-H3, with the latter being the predominant form.
Some studies have shown that B7-H3 exhibits both co-stimulatory and inhibitory effects on the immune system in non-malignant tissues. On one hand, B7-H3 acts as a co-stimulatory factor, enhancing T cell activation, proliferation, and cytokine secretion (such as IFN-γ and IL-2). On the other hand, B7-H3 can function as a co-inhibitory molecule in certain adaptive immune responses, enabling tumors to evade immune detection and suppress NK cell–mediated cytolysis.
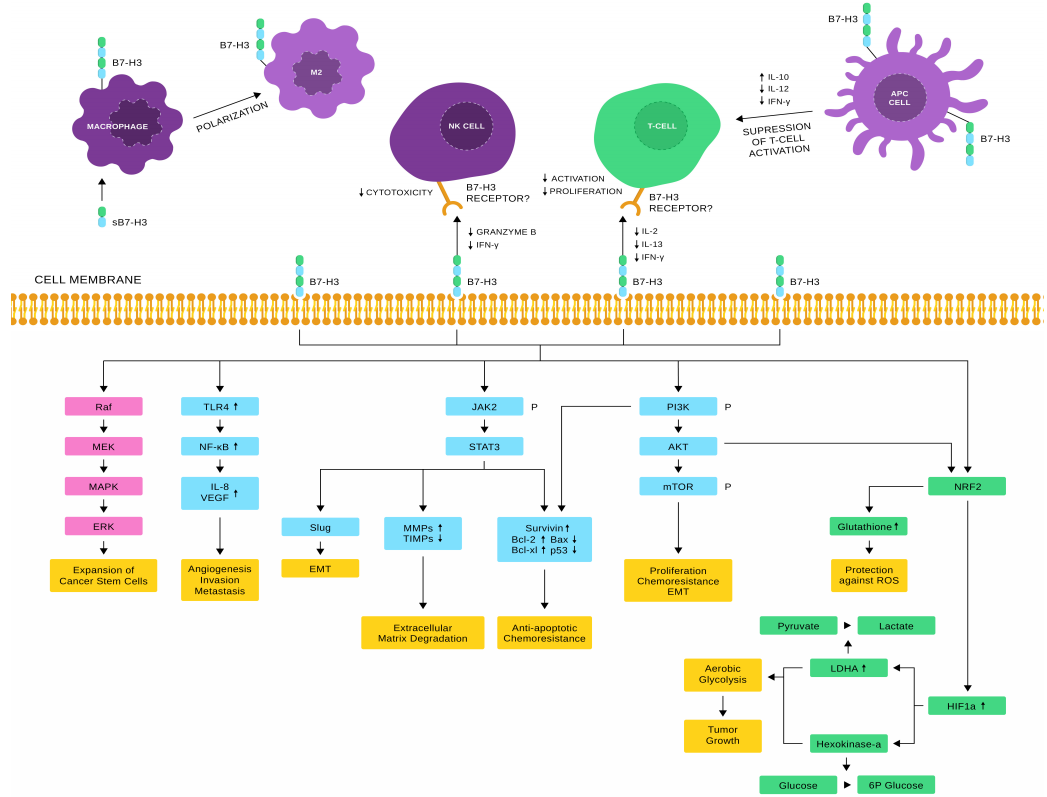
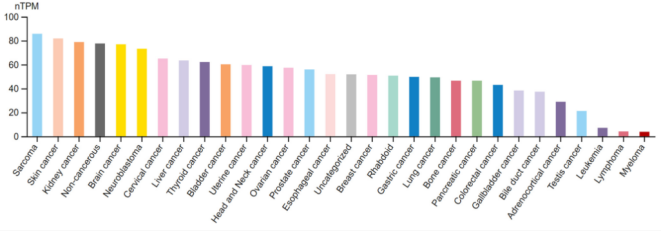
Studies have found that B7-H3 is overexpressed in a variety of tumor tissues, including non-small cell lung cancer, pancreatic cancer, and primary liver cancer. Excessive expression of B7-H3 in tumors is often associated with poor patient prognosis, shorter overall survival, and reduced progression-free survival. In lung cancer, particularly small cell lung cancer, approximately 65% of patients exhibit high B7-H3 expression in their tumors, which is correlated with unfavorable outcomes, making B7-H3 a promising therapeutic target.
Recent Advances in B7-H3–Targeted Therapy
Based on the high expression of B7-H3 in tumor tissues, antibody–drug conjugates (ADCs) have become the most representative approach in development. The core design of B7-H3 ADCs involves using anti–B7-H3 monoclonal antibodies to achieve specific recognition of tumor cells. Upon internalization, the ADC releases highly potent cytotoxic payloads—such as topoisomerase I inhibitors or microtubule inhibitors—enabling precise killing of tumor cells.
Globally, B7-H3–targeted therapies under investigation are still dominated by ADCs, while gradually expanding into novel molecular formats, including bispecific fusion proteins.
Although no B7-H3 ADC has yet been approved for clinical use, investment in research and clinical exploration targeting this antigen remains strong. Companies worldwide continue to make persistent efforts to overcome this challenging target.
According to incomplete data from Pharma Research Network, among the global B7-H3 ADC pipeline, four candidates have entered Phase III clinical trials, three of which are from China: Qilu Pharma’s MHB088C, MediLink Biotech’s YL201, and Hansoh Pharmaceutical’s HS-20093.
Existing clinical data indicate that B7-H3 ADCs show significant potential across multiple indications, including extensive-stage small cell lung cancer (ES-SCLC), nasopharyngeal carcinoma (NPC), sarcomas, gynecological tumors, and brain metastases, with their therapeutic scope continuing to expand.
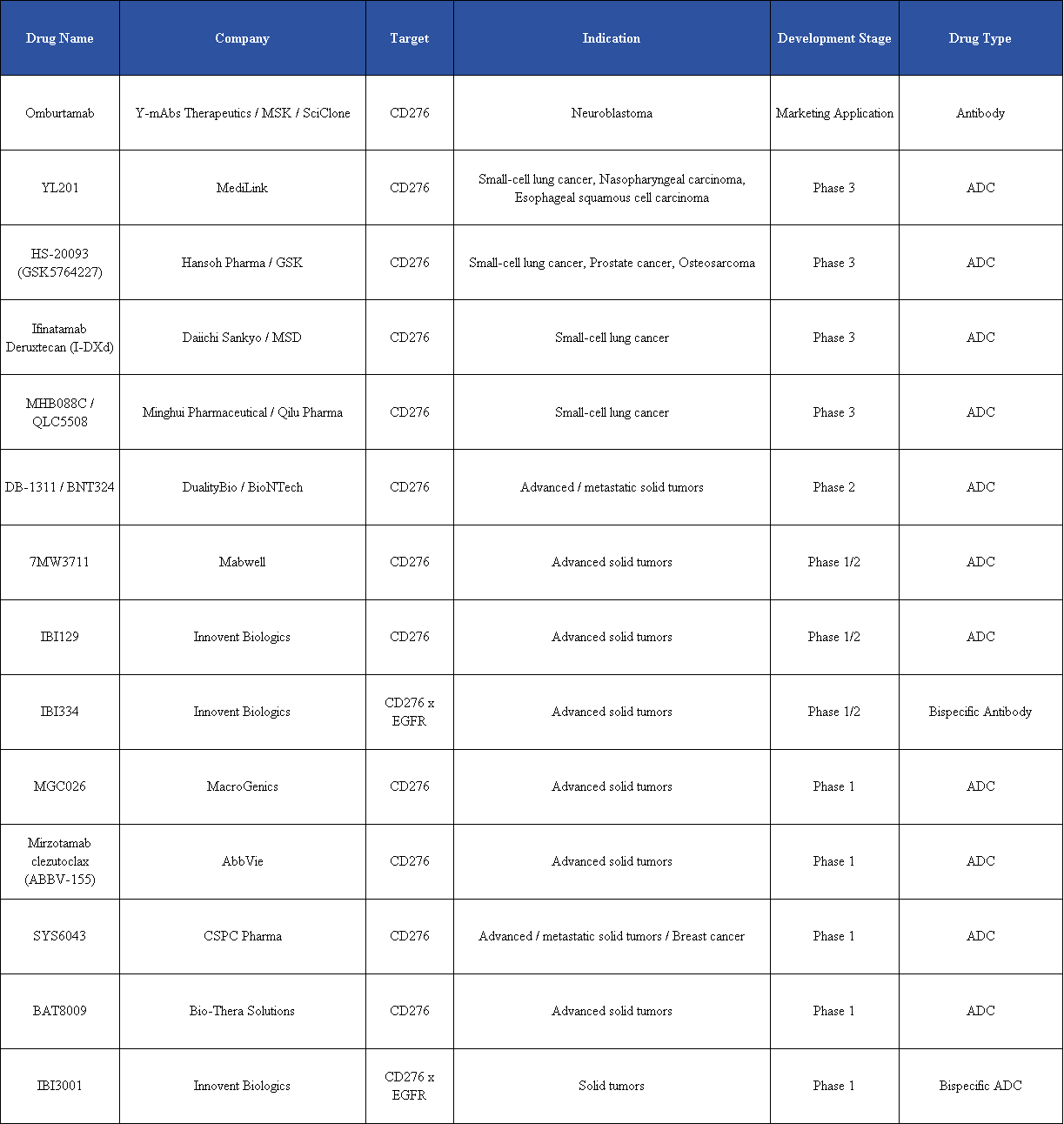
On the international stage, inatamab deruxtecan (I-DXd), co-developed by Daiichi Sankyo and Merck, has emerged as a leading example in the B7-H3 ADC field. This drug has entered a global Phase III clinical trial in prostate cancer, making it the world’s first B7-H3 ADC to reach Phase III. Built on Daiichi Sankyo’s proprietary DXd platform, I-DXd signals an intensifying phase of global competition in B7-H3 ADC development.
However, the development of B7-H3 ADCs has faced challenges. Due to lung toxicity safety signals, certain Phase III lung cancer trials of I-DXd were previously placed on hold by regulators. Recently, the FDA lifted partial trial restrictions, providing a significant boost to a field that had been temporarily stalled and offering important regulatory guidance for similar programs.
Several companies in Asia have also advanced B7-H3 ADCs into late-stage clinical trials. MediLink, using its innovative TMALIN® platform, has developed YL201, which previously received FDA Breakthrough Therapy designation for small cell lung cancer (SCLC). The YL201 program is currently conducting multiple clinical studies worldwide. For SCLC and nasopharyngeal carcinoma (NPC), Phase III trials are ongoing in China, while additional monotherapy and combination studies are exploring its potential across a broad range of solid tumors. Early clinical data show approximately 40% objective response rate (ORR) in Phase I/II trials, positioning YL201 as a representative ADC program with global aspirations.
Hansoh Pharmaceutical’s HS-20093 is advancing multiple Phase III trials and has licensed overseas rights to GSK, also receiving FDA Breakthrough Therapy designation. At the 2025 ESMO meeting, Phase II data demonstrated that the 12 mg/kg Q3W dose showed manageable safety and preliminary efficacy in relapsed/refractory osteosarcoma and soft tissue sarcoma, with ORR of 28.6% and 25%, respectively, and disease control rates (DCR) of 85.7% for osteosarcoma—addressing an unmet need in sarcoma treatment.
Minghui Pharmaceutical’s innovative B7-H3 ADC, developed on the SuperTopoi™ platform, combines enhanced tumor-killing potency with improved safety, significantly widening the therapeutic window. Strategic collaborations, such as the BD deal with Qilu Pharmaceutical, have accelerated its entry into Phase III.
7MW3711, developed by Mabwell Biotech, has also disclosed clinical data at international conferences including ASCO 2025, demonstrating the capability of its ADC platform.
Combination strategies are emerging as another key competitive direction for B7-H3 ADCs. Examples include YL201 combined with PD-1/VEGF bispecific antibodies (Akeso Biopharma), DB-1311 combined with BioNTech immunotherapy, and 7MW3711 combined with PD-1 inhibitors—all currently in clinical evaluation. Such diversified combination approaches aim to deepen efficacy and expand indications, consolidating strategic positions within the B7-H3 ADC field.
Summary
With multiple B7-H3 ADC pipelines entering late-stage clinical development, a new wave of global competition around this target has emerged. Whether through differentiated payload design, such as Exatecan-based ADCs, or synergistic combinations with PD-(L)1 inhibitors, the therapeutic potential of B7-H3 is transitioning from exploratory evidence toward clinical validation. The next major “blockbuster ADC” may well emerge from this target space.
Reference
Y-mAbs Therapeutics, Inc. (2020, August 6). Y-mAbs announces completion of submission of omburtamab biologics license application to FDA. Y-mAbs Therapeutics, Inc. News Releases. https://ir.ymabs.com/news-releases/news-release-details/y-mabs-announces-completion-submission-omburtamab-biologics/
BioNTech SE. (2023, April 3). BioNTech and DualityBio form global strategic partnership to accelerate development of differentiated antibody‑drug conjugate therapeutics for solid tumors. BioNTech Investors. https://investors.biontech.de/news-releases/news-release-details/biontech-and-dualitybio-form-global-strategic-partnership/
Daiichi Sankyo U.S. (2025, August 18). Ifinatamab deruxtecan granted Breakthrough Therapy Designation by U.S. FDA for patients with pretreated extensive‑stage small cell lung cancer. Daiichi Sankyo U.S. Press Releases. https://daiichisankyo.us/press-releases/-/article/ifinatamab-deruxtecan-granted-breakthrough-therapy-designation-by-us-fda-for-patients-with-pretreated-extensive-stage-small-cell-lung-cancer
Ellipses Life. (2025, December 20). Ellipses in licenses: First-in-class B7H3 antibody-drug conjugate from China. https://ellipses.life/ellipses-in-licenses-first-in-class-b7h3-antibody-drug-conjugate-from-china/
GSK plc. (2023, December 20). GSK enters exclusive license agreement with Hansoh for HS‑20093. GSK Media. https://www.gsk.com/en-gb/media/press-releases/gsk-enters-exclusive-license-agreement-with-hansoh-for-hs-20093/
Roche returns to MediLink with promise of $570M near term payments for another ADC. (2026, January 9). FierceBiotech. https://www.fiercebiotech.com/biotech/roche-returns-medilink-promise-570m-near-term-payments-another-adc
ClinicalTrials.gov. (n.d.). NCT06612151. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06612151
ClinicalTrials.gov. (n.d.). NCT06498479. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06498479
ClinicalTrials.gov. (n.d.). NCT06925737. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06925737
ClinicalTrials.gov. (n.d.). NCT06954246. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06954246
ClinicalTrials.gov. (n.d.). NCT06892548. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06892548
ClinicalTrials.gov. (n.d.). NCT06008366. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06008366
ClinicalTrials.gov. (n.d.). NCT05991349. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT05991349
ClinicalTrials.gov. (n.d.). NCT05774873. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT05774873
ClinicalTrials.gov. (n.d.). NCT06242470. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06242470
ClinicalTrials.gov. (n.d.). NCT03595059. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT03595059
ClinicalTrials.gov. (n.d.). NCT07241936. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT07241936
ClinicalTrials.gov. (n.d.). NCT05405621. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT05405621
ClinicalTrials.gov. (n.d.). NCT06349408. U.S. National Library of Medicine. https://www.clinicaltrials.gov/study/NCT06349408