In the first half of 2025, AstraZeneca’s financial report showed that its blockbuster respiratory and immunology product Tezspire (TSLP monoclonal antibody) achieved a year-on-year sales growth of 73%, driving an overall company performance increase of 11% and emerging as a dark horse in the global autoimmune disease field.
TSLP Target
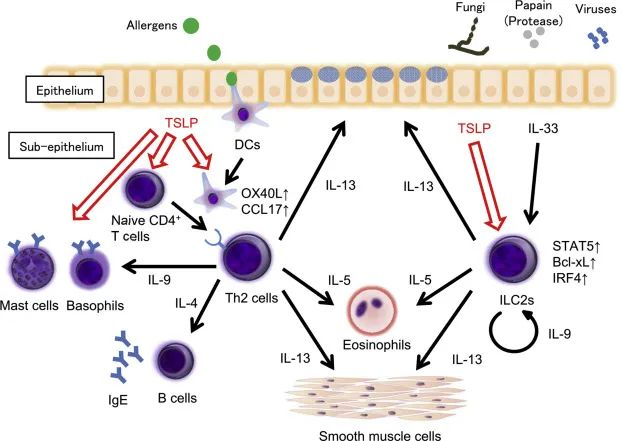
The TSLP/TSLPR signaling pathway plays a crucial role in regulating immune responses, especially in allergic and autoimmune diseases. Additionally, this pathway is involved in modulating T cell polarization, dendritic cell activation, and antigen presentation functions of immune cells.Learn more about our catalog.
Thymic stromal lymphopoietin (TSLP) is an epithelial cytokine that plays a key role in the initiation and persistence of airway inflammation. TSLP can drive the release of downstream type 2 (T2) cytokines, including IL-4, IL-5, and IL-13, leading to asthma and other inflammatory symptoms. At the same time, TSLP can activate multiple cell types involved in non-T2-driven inflammation.
Two isoforms of TSLP exist in human tissues: the short form (sfTSLP), composed of 63 amino acids and primarily expressed under homeostatic conditions, which is related to the steady-state functions of TSLP; and the long form (lfTSLP), consisting of 159 amino acids, which can be upregulated by Toll-like receptor ligands, pro-inflammatory cytokines, specific cellular cytokine environments, and TNF-α alone.
Expression of the long isoform occurs upstream of multiple inflammatory cascades and mediates the overactivated immune responses seen in various allergic diseases. It has been confirmed to play a significant role in type 2 immune responses.
The TSLP receptor (TSLPR) belongs to the hematopoietic cytokine receptor family and is a type I cytokine receptor protein. TSLPR forms a high-affinity receptor complex only when paired with the IL-7 receptor α chain (IL-7Rα), as its affinity for TSLP alone is very low.
Studies have shown that glucocorticoids can reduce the expression of TSLP and TSLPR in asthmatic mice, altering TSLP-induced dendritic cell (DC) functions. This shifts antigen-induced Th2 responses toward Th1 responses, decreasing IL-4, IL-5, and IL-13 levels while increasing IL-10 and IFN-γ production. This effect depends on the downregulation of OX40L and increased IL-12 production in DCs. Research in asthma patients indicates a positive correlation between the severity of airway inflammation and TSLP expression levels.
The TSLP-TSLPR axis and its downstream signaling molecules play vital roles in the pathogenesis and progression of allergic inflammatory diseases, such as allergic asthma.
TSLP’s Potential Is Emerging with a Broad Future
Currently, TSLP has been confirmed to be associated with various allergic diseases, including atopic dermatitis, bronchial asthma, and eosinophilic esophagitis. Additionally, literature indicates that TSLP is also related to chronic inflammatory diseases such as chronic obstructive pulmonary disease (COPD) and celiac disease, as well as autoimmune diseases like psoriasis and rheumatoid arthritis.
TSLP-targeting drugs not only possess broad-spectrum characteristics capable of effectively addressing multiple indications, but these indications also have wide-ranging impacts and prolonged disease courses, conferring high commercial value.
Due to its enormous market potential, the TSLP target has attracted increasing attention from pharmaceutical companies. On November 18, 2024, Biosion announced the licensing of the global rights outside Greater China for its TSLP antibody BSI-045B and TSLP/IL-4R bispecific antibody to Aclaris Therapeutics, receiving over $40 million in upfront cash payments, more than $900 million in milestone payments, and a 19.9% equity stake in Aclaris Therapeutics.
Moreover, at the beginning of 2024, Aiolos Bio, acquired by GSK, licensed its sole pipeline asset—the TSLP monoclonal antibody AIO-001—for a total deal value of $1.4 billion, with a $1 billion upfront payment.
R&D Landscape of the Target
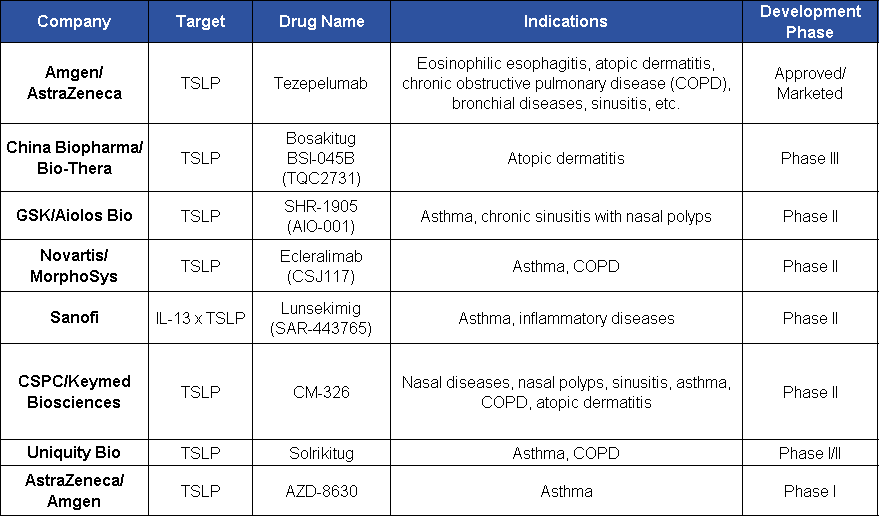
As an important target in inflammatory and autoimmune diseases, TSLP has seen the fastest clinical progress in asthma indications. Due to the association between COPD and asthma, research on the use of TSLP monoclonal antibodies for COPD treatment is also actively advancing. Currently, only one TSLP monoclonal antibody — Tezspire (developed by Amgen/AstraZeneca) — has been approved and marketed, with global sales exceeding $1.2 billion in 2024.
In addition, TQC2731 (BSI-045B) is the fastest progressing TSLP monoclonal antibody in development and has entered Phase III clinical trials.
Tezspire
Tezspire (Tezepelumab) is the first approved anti-TSLP monoclonal antibody (brand name Tezspire) indicated for severe or refractory asthma. Multiple registrational Phase III studies are underway to expand its indications, such as chronic rhinosinusitis with nasal polyps (CRSwNP), with positive key Phase III data already published. It received FDA approval in December 2021 for the treatment of severe asthma in patients aged 12 years and older.
In 2024, Tezspire’s sales surpassed $1 billion for the first time, reaching $1.219 billion. In the first half of 2025, AstraZeneca’s financial report did not disclose Tezspire’s exact sales figures but confirmed that sales growth reached 73%, driving an overall company revenue increase of 11%. According to Waypoin data, Leerink forecasts Tezspire’s sales to reach $4.3 billion by 2030, up from prior industry estimates of $3 billion.
Additionally, Tezepelumab is being investigated for COPD indications. It has completed Phase II clinical trials and is currently in Phase III development. On February 25, 2025, the CDE (Center for Drug Evaluation) website showed that AstraZeneca and Amgen jointly submitted a new indication marketing application for Tezepelumab injection. Based on its clinical development progress, the submitted indication is speculated to be chronic rhinosinusitis with nasal polyps.
SHR-1905
SHR-1905 is a TSLP monoclonal antibody independently developed by Hengrui and protected by intellectual property rights. In August 2023, Hengrui reached an agreement with the U.S.-based company One Bio (later renamed Aiolos Bio) to license the SHR-1905 injection project to them for a fee. The upfront payment and near-term milestone payments totaled $25 million, with research and development as well as sales milestone payments potentially reaching up to $1.025 billion. In early 2024, GSK acquired Aiolos Bio for $1.4 billion, thereby obtaining the related rights to this product.
TQC2731 (BSI-045B)
BSI-045B (TQC2731) was originally developed by Biosion, with multiple clinical studies in China being advanced by Biosion’s Greater China partner, CSPC Pharmaceutical Group. Notably, in November this year, Biosion and Aclaris Therapeutics reached an exclusive global licensing agreement (excluding Greater China) for two TSLP-targeting drugs, including this molecule.
Currently, BSI-045B (TQC2731) is conducting Phase III clinical trials in China and Phase II trials globally, targeting indications such as asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), atopic dermatitis, and chronic obstructive pulmonary disease (COPD). In July, the Chinese Clinical Trial Registry showed that CSPC Pharmaceutical Group registered a Phase III clinical trial of TQC2731 injection for patients with CRSwNP.
In the United States, TQC2731 completed a Phase IIa single-arm proof-of-concept clinical trial involving 22 patients with moderate-to-severe atopic dermatitis. The study results demonstrated excellent pharmacodynamics, safety, and efficacy data, supporting the potential of this drug molecule as a first-in-class therapy for atopic dermatitis.
Summary
The TSLP/TSLPR axis is a highly valuable therapeutic target, especially in the treatment of allergic and inflammatory diseases. Leveraging its strong expertise in cell engineering and protein technologies, Genomeditech focuses on supporting TSLP-targeted drug development by providing stable overexpressing cell lines, reporter gene assay cell lines, and high-quality antibodies and protein products, all functionally validated for reliability and stability. This empowers research and industry partners to accelerate translational progress and drive innovation in allergy and inflammatory disease therapies.
Reference
AstraZeneca. (2025, April 29). Q1 2025 results announcement. https://www.astrazeneca.com/content/dam/az/PDF/2025/q1/Q1-2025-results-announcement.pdf
Biosion. (2025, April 29). Biosion today presented key phase 2 POC clinical study results for Bosakitug, an anti-TSLP mAb, in atopic dermatitis subjects at the Revolutionizing Atopic Dermatitis Conference. PR Newswire. https://www.prnewswire.com/news-releases/biosion-today-presented-key-phase-2-poc-clinical-study-results-for-bosakitug-an-anti-tslp-mab-in-atopic-dermatitis-subjects-at-the-revolutionizing-atopic-dermatitis-conference-302168770.html
ClinicalTrials.eu. (2023). Solrikitug – Application in Therapy and Current Clinical Research. https://clinicaltrials.eu/inn/solrikitug/
GSK. (2024, January 9). GSK enters agreement to acquire Aiolos Bio. https://www.gsk.com/en-gb/media/press-releases/gsk-enters-agreement-to-acquire-aiolos-bio
Sanofi. (2025, April 15). Sanofi’s respiratory pipeline advances with new data in asthma and plans for new clinical studies in COPD. https://www.sanofi.com/en/media-room/press-releases/2025/2025-04-15-05-00-00-3061368
U.S. Food and Drug Administration. (2021, December 17). FDA approves maintenance treatment for severe asthma. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-maintenance-treatment-severe-asthma
U.S. National Library of Medicine. (2020). Study: Ecleralimab (CSJ117). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04410523
U.S. National Library of Medicine. (2023). Study [NCT05110976]. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT05110976
U.S. National Library of Medicine. (2023). Study [NCT06372678]. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT06372678