In the realm of primary sclerosing cholangitis (PSC), a rare but aggressively progressive cholangiopathy, a groundbreaking study published in August 2025 in Journal of Hepatology has garnered worldwide attention. The research from Inserm U1110, University of Strasbourg, titled “Claudin-1 is a mediator and therapeutic target in primary sclerosing cholangitis,” is the first to definitively implicate CLDN1 as a central driver in PSC pathogenesis and to validate its potential as a therapeutic intervention.
Remarkably, Alentis Therapeutics remains the only company worldwide advancing CLDN1-targeted therapies, including monoclonal antibodies and ADCs, into the clinical stage. This development raises an important question for the global pharmaceutical community: Should CLDN1 be prioritized as a promising target for future research and drug development?
The key issue now is: What sets CLDN1 apart? Why does this protein play pivotal roles across multiple disease states, and what underlies its broad therapeutic potential?
1. Background of CLDN-1
Claudin-1 (CLDN1) is a key epithelial tight junction protein that plays a central role in maintaining the integrity of various tissue barriers. This protein contains four transmembrane domains and assembles into tight junction strands by forming homo- or heterodimers with other claudin family members. Through this structural organization, it effectively seals intercellular spaces and regulates paracellular permeability to water, ions, and solutes. CLDN1 is therefore essential for preserving the structural and functional integrity of epithelial barriers in tissues such as the skin, gastrointestinal tract, liver, and kidney.
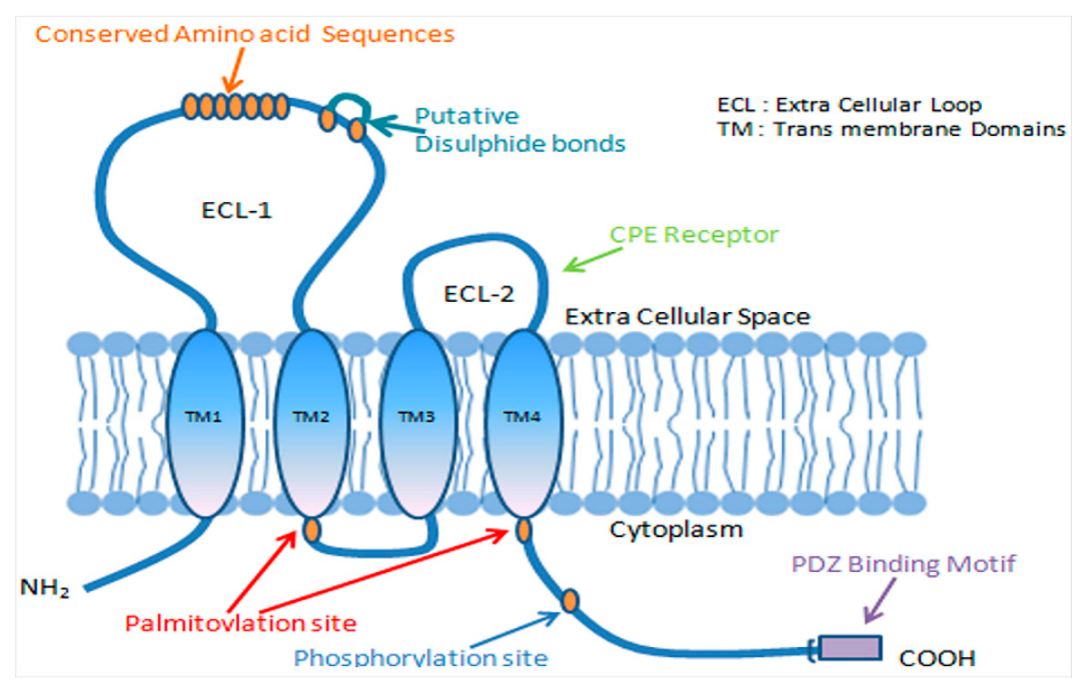
Under normal physiological conditions, the expression and integrity of CLDN1 are regulated by transcription factors, growth factors, and cytokines, ensuring proper gating and barrier functions of tight junctions. Dysregulation of CLDN1 can impair both barrier integrity and gating function, leading to upregulation of pro-inflammatory markers such as IFN-γ and TNF-α. In cancer, alterations in CLDN1 expression are closely associated with tumor type and subtype.
2. Therapeutic Potential of Claudin-1 in Disease
2.1. Claudin-1 and Cancer: Tumor Promoter or Suppressor
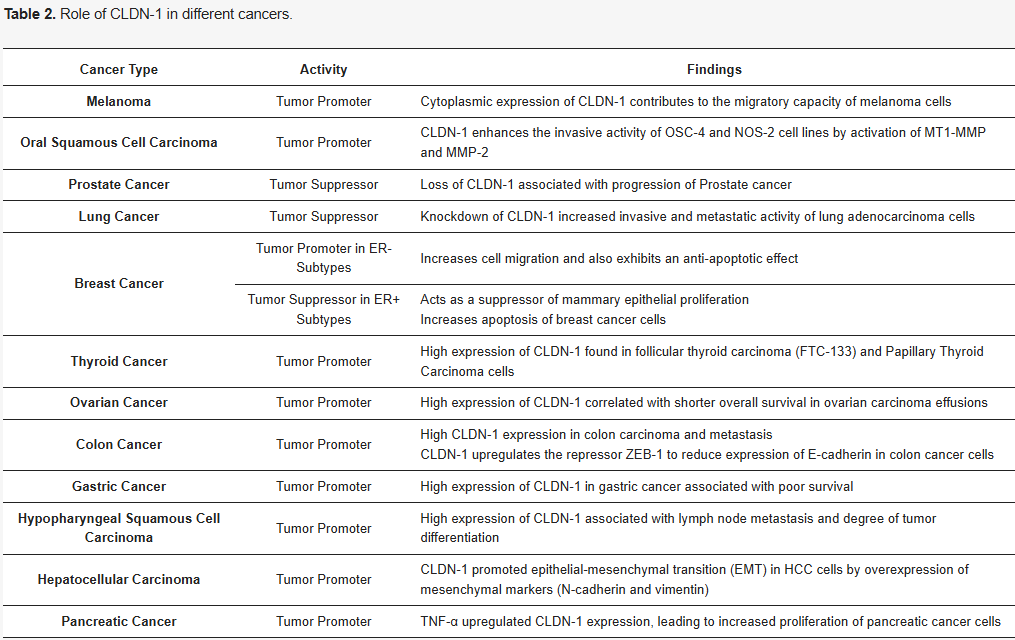
CLDN1 plays a complex and pivotal role in the initiation and progression of various cancers. Its expression levels vary markedly across tumor types: in malignancies such as breast cancer, esophageal cancer, and prostate cancer, CLDN1 is often downregulated, whereas in colorectal cancer, ovarian cancer, and oral squamous cell carcinoma, overexpression is frequently observed. These abnormal expression patterns are closely associated with tumor cell migration, invasion, and metastasis, suggesting that CLDN1 may exert tissue-specific regulatory effects on tumor progression. Although the precise mechanisms remain incompletely understood, the expression profile of CLDN1 has emerged as a potential biomarker for prognostic assessment in multiple cancer types.![]()
2.2 Regulatory Role of Claudin-1 in Inflammatory Diseases
Beyond its overexpression in various cancers, abnormal expression or dysfunction of Claudin-1 is closely associated with multiple inflammatory conditions. For example, in inflammatory bowel disease (IBD), downregulation of Claudin-1 can impair the intestinal epithelial barrier, increasing permeability and allowing bacteria and antigens to translocate into the lamina propria, thereby triggering and sustaining inflammatory responses. In skin disorders such as atopic dermatitis, loss of Claudin-1 disrupts epidermal barrier function, facilitating allergen penetration and water loss.
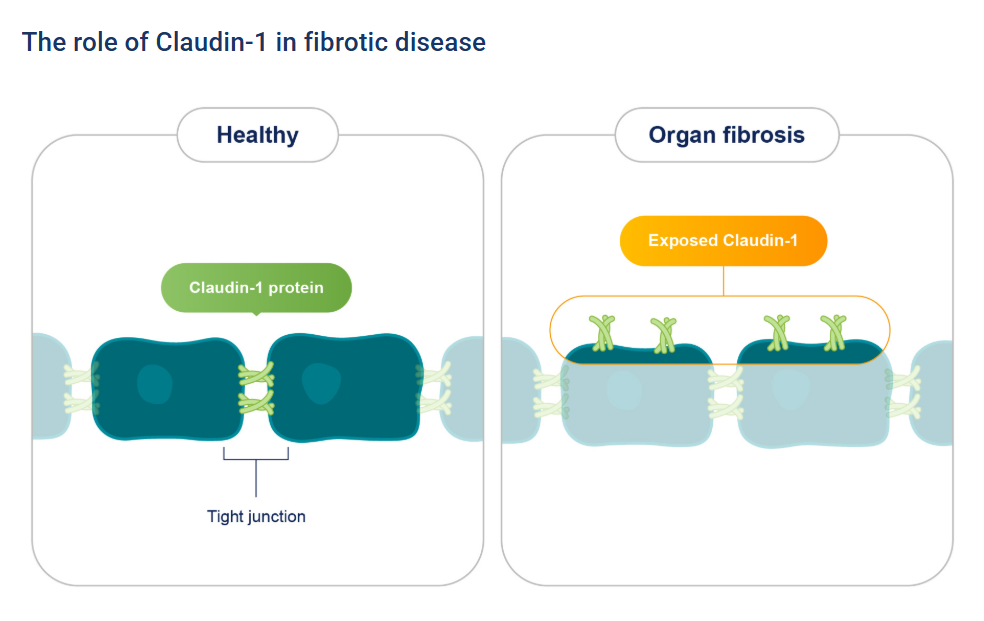
2.3 Role of Claudin-1 in Fibrotic Diseases and Therapeutic Prospects
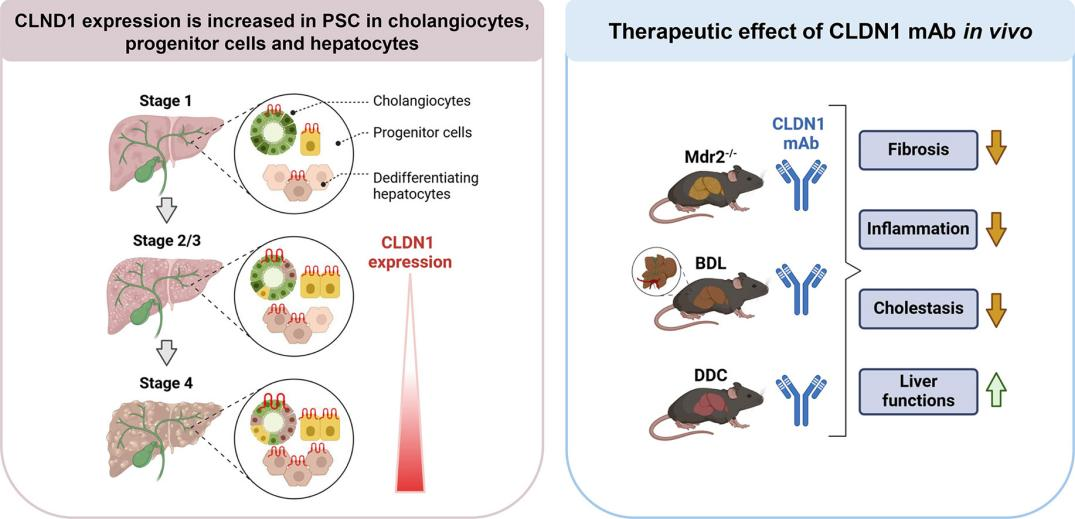
Recent research, titled “Claudin‑1 is a mediator and therapeutic target in primary sclerosing cholangitis,” has further demonstrated that CLDN1 plays a key role in fibrotic diseases such as primary sclerosing cholangitis (PSC). PSC is a chronic progressive cholangiopathy characterized by bile duct fibrosis, inflammatory responses, and risk of malignant transformation.
In this study, the researchers analyzed liver tissue samples from PSC patients using single-cell RNA sequencing, spatial transcriptomics, and multi-omics proteomics. They found that CLDN1 was significantly upregulated in cholangiocytes and cholestasis-associated hepatocytes, and its expression levels positively correlated with disease progression. In three classical PSC animal models (bile duct ligation [BDL], DDC feeding, and Mdr2^-/- mice), treatment with a CLDN1-specific monoclonal antibody markedly improved liver function, reduced liver fibrosis and cholestasis, and increased survival. Conditional knockout of CLDN1 in hepatic epithelial cells similarly attenuated liver injury and collagen deposition.
These findings provide compelling evidence that CLDN1 plays a central role in PSC pathogenesis and establish a strong scientific foundation for developing novel CLDN1-targeted therapies for this disease.
Given its central role in disease mechanisms, Claudin-1 has emerged as a promising therapeutic target. Current research strategies include the development of Claudin-1-targeted monoclonal antibodies to block its deleterious effects in inflammation and cancer, modulation of its expression or function using small molecules or nucleic acid–based therapies, and exploration of gene therapy approaches to restore normal Claudin-1 expression in barrier-related disorders. These interventions offer new therapeutic avenues for inflammatory bowel disease, certain skin disorders, and malignancies.
3. Current Status of Claudin-1-Targeted Therapeutics
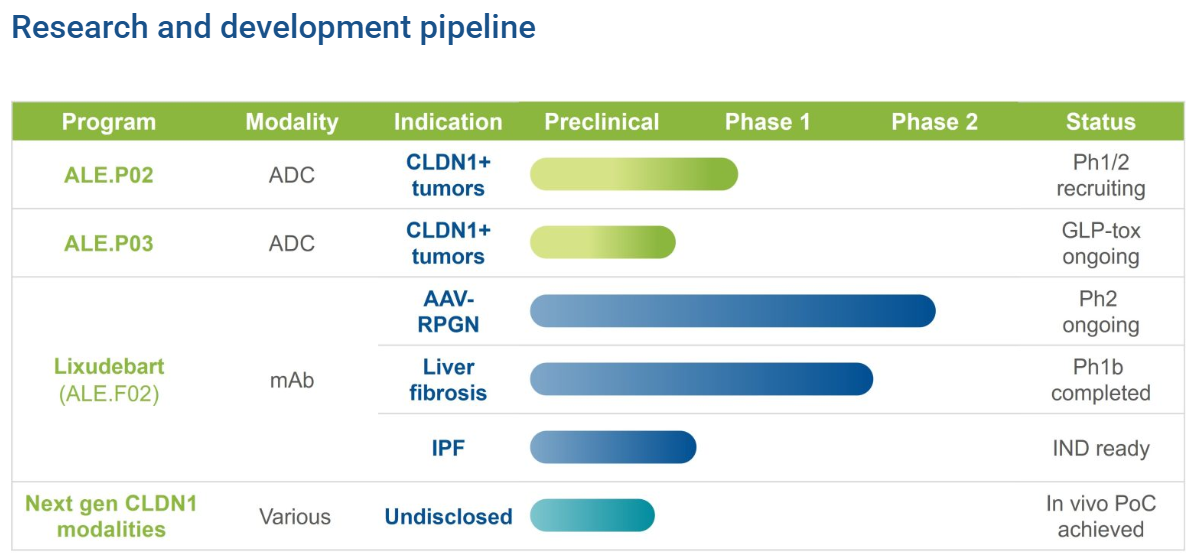
In the development of Claudin-1-targeted therapies, Alentis Therapeutics has taken the lead by employing anti-CLDN1 antibody–drug conjugates (ADCs) and monoclonal antibodies for the treatment of cancer and fibrotic diseases. Among these, the CLDN1 antibody Lixudebart (ALE.F02) from Alentis Therapeutics has shown the most rapid progress in clinical development.
3.1. CLDN1 mAb
Lixudebart (ALE.F02) is a first-in-class monoclonal antibody primarily developed for fibrotic lesions in the liver, kidney, and lungs. Its therapeutic indications include ANCA-associated vasculitis with renal involvement, advanced liver fibrosis, and idiopathic pulmonary fibrosis (IPF). The drug is designed to reverse fibrosis and protect or restore organ function by blocking fibrotic signaling pathways and promoting collagen barrier remodeling.
To date, Lixudebart has completed Phase I single- and multiple-dose escalation studies in healthy volunteers and a Phase Ib study in patients with advanced liver fibrosis, yielding encouraging preliminary results. Additionally, a Phase I/II clinical trial targeting ANCA-associated vasculitis with renal involvement (ClinicalTrials.gov Identifier: NCT06047171) is currently ongoing, with mid-term data highly anticipated. The development team plans to advance Lixudebart into Phase II clinical studies for the treatment of IPF in the next stage of research.
3.2. CLDN1 ADCs
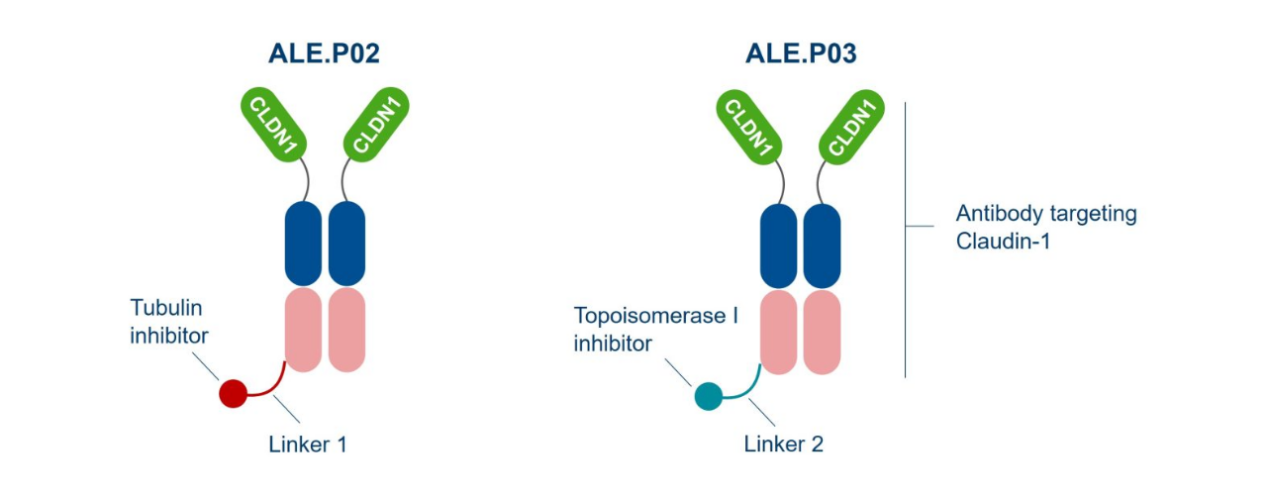
In addition to monoclonal antibodies, Alentis Therapeutics is developing CLDN1-targeted antibody–drug conjugates (ADCs). ALE.P02 and ALE.P03 are first-in-class ADCs designed for CLDN1-positive (CLDN1⁺) cancer indications. These ADCs aim to selectively deliver potent anticancer agents directly to CLDN1⁺ tumor tissues while sparing healthy tissues.
Currently, ALE.P02 has received FDA Fast Track designation. A Phase I/II study of ALE.P02 in CLDN1⁺ squamous solid tumors (ClinicalTrials.gov Identifier: NCT06747585) is actively recruiting patients. Meanwhile, the first-in-human clinical trial for ALE.P03 targeting CLDN1⁺ solid tumors is under planning.
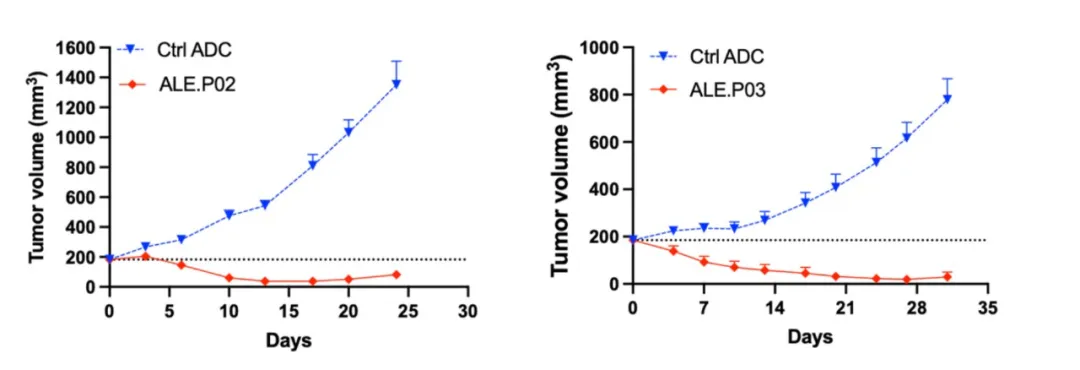
Preclinical studies of ALE.P02 and ALE.P03 have demonstrated that both ADCs selectively bind to and are internalized by CLDN1-positive tumor cells. Across various in vivo tumor models and different levels of CLDN1 expression, these ADCs exhibited significant tumor growth inhibition.
4. Summary
Claudin-1 is not only a critical molecular component for epithelial barrier function, but its dysregulation also contributes to the pathogenesis of various diseases. Therefore, therapeutic strategies targeting this protein hold broad potential for clinical translation.
Reference
Bhat, A. A., Syed, N., Therachiyil, L., Nisar, S., Hashem, S., Macha, M. A., Yadav, S. K., Krishnankutty, R., Muralitharan, S., Al-Naemi, H., Bagga, P., Reddy, R., Dhawan, P., Akobeng, A., Uddin, S., Frenneaux, M. P., El-Rifai, W., & Haris, M. (2020). Claudin-1, A Double-Edged Sword in Cancer. International Journal of Molecular Sciences, 21(2), 569. https://doi.org/10.3390/ijms21020569
Del Zompo, F., Crouchet, E., Ostyn, T., Nehme, Z., Messé, M., Juehling, F., Désert, R., Vieira, A. T., Moehlin, J., Nakib, D., Andrews, T., Perciani, C., Chung, S., Bader, G., McGilvray, I., Caime, C., Scaravaglio, M., Carbone, M., Invernizzi, P., . . . Baumert, T. F. (2025). Claudin-1 is a mediator and therapeutic target in primary sclerosing cholangitis. Journal of Hepatology. https://doi.org/10.1016/j.jhep.2025.08.005
Ruwisch, J., Jäger, B., Terwolbeck, O., Teixeira, G., Juehling, F., Baumert, T. F., & Prasse, A. (2022). Beyond barrier function – claudin-1 as a therapeutic target candidate for idiopathic pulmonary fibrosis. 03.02 - Airway Cell Biology and Immunopathology, 4508. https://doi.org/10.1183/13993003.congress-2022.4508
Alentis Therapeutics. (2025). Pipeline - Alentis Therapeutics. https://alentis.ch/pipeline/